INTRODUCTION
Colonic atresia (CA) is a rare cause of congenital intestinal obstruction. It accounts for 5-15% of bowel atresia in neonates. The incidence is about 1 in 60,000 births.[1] The average of reported cases is about 1 to 2 cases per year in a tertiary center worldwide.[2] As it is rarely encountered, the management of CA is challenging and controversial. Various approaches have been suggested, including the staged procedure versus the single-stage procedure.
One of the predicting factors for surgical decision is the site of colonic atresia. Right-sided CA has a relatively shorter length and thicker proximal colon which is grossly dilated, worse in the case of the competent ileocecal valve. This carries a huge discrepancy for a primary colo-colic anastomosis and imposes a higher risk of anastomotic leak. Option of the primary colo-colic anastomosis with a proximal stoma has been reported to reduce the risk of anastomotic leak.[3] Some authors preferred to sacrifice the dilated colonic segment and had ileocolic anastomosis.[4], [5] In this report, we are sharing a case of right-sided CA and a review of the reported series on right-sided CA to study the feasibility of primary anastomosis vs. staged procedure.
Case Report
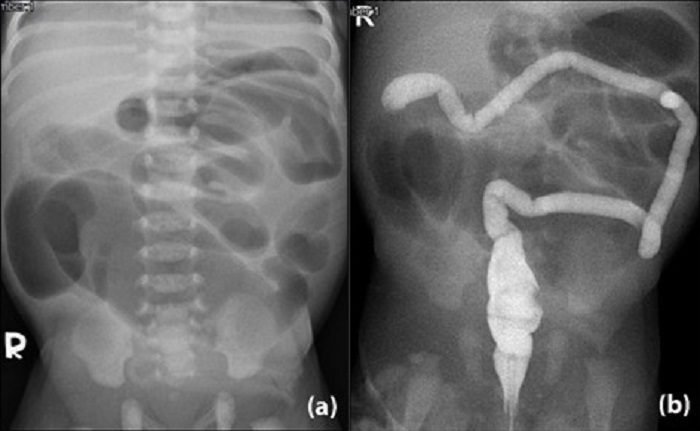
A full-term male baby weighing 3006 gm. was born of a 27-year-old mother who had a history of Hepatitis B and Chlamydial trachomatis infections. There were no antenatal visits. Breastfeeding was started soon after birth, however, he developed abdominal distension and bilious vomiting. His first stool (at day 1 of life) was a minimal whitish plug rather than meconium. Clinically, the baby was active and non-septic. The abdomen was distended with visible bowel loops. It was not tense nor discolored. The anus was at a normal position. A plain abdominal radiograph showed dilated bowel loops, absence of rectal gas, and no pneumoperitoneum (Fig. 1a). Contrast enema showed features of microcolon, but the contrast was unable to pass beyond hepatic flexure (Fig. 1b).
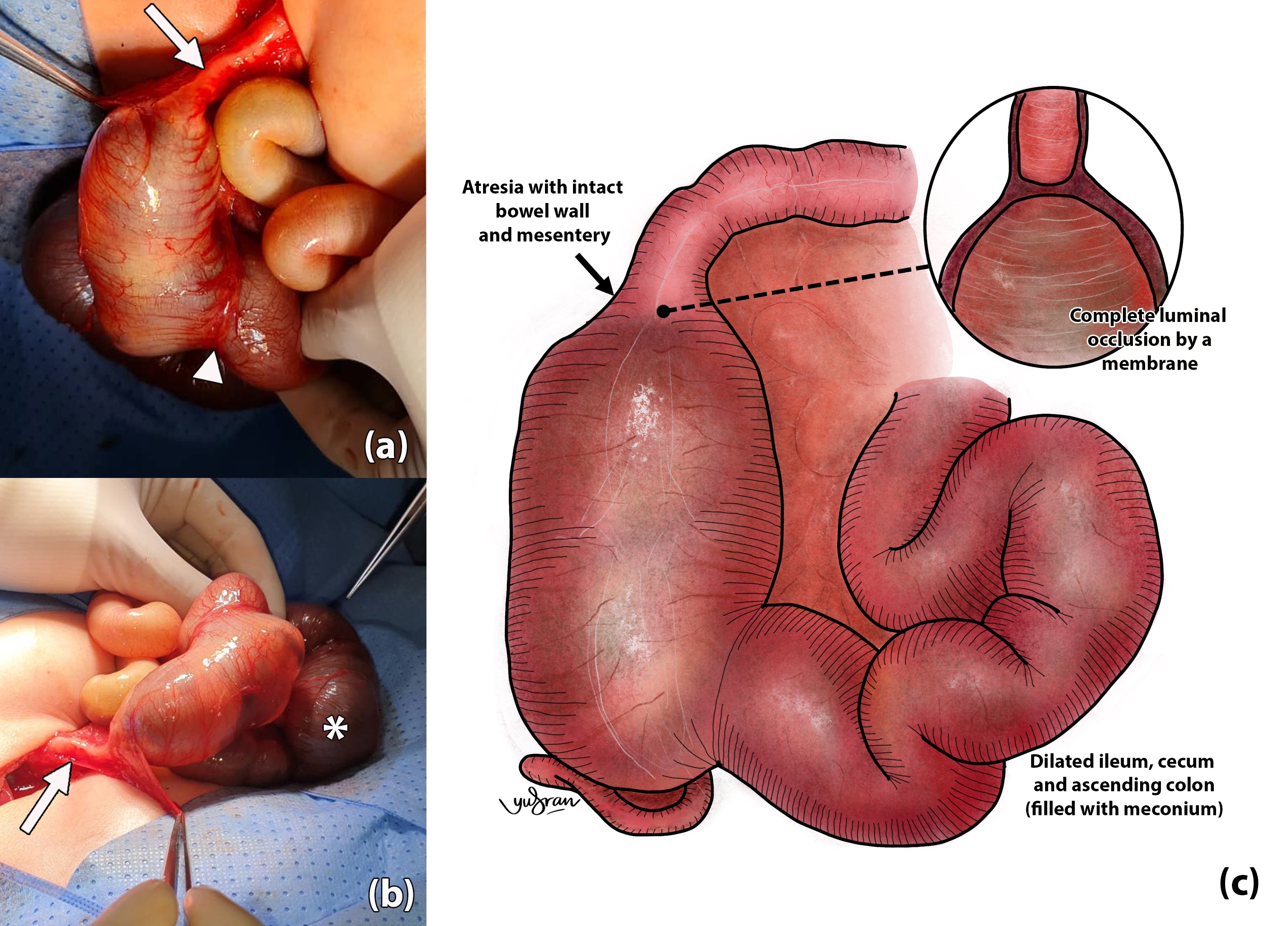
The baby underwent laparotomy on day 3 of life and was intraoperatively noted to have a type 1 colonic atresia at the ascending colon, 7cm from the ileocecal valve (Fig. 2). Both proximal colon and distal ileum were grossly dilated. Upon resection at the level of the atresia, the ileocecal valve was seen wide open, and bowel decompression, including from the distal ileum, was easily done from the opening. Once decompressed, a reasonable discrepancy for primary anastomosis was noted; estimated at 3 to 4:1. A cheatle slit was performed at the distal segment followed by primary anastomosis. Few interrupted seromuscular tapering sutures were applied at the dilated proximal colon (the antimesenteric border was folded inward) using 4-0 braided synthetic absorbable sutures about 1 to 2 cm apart. As the contrast enema has shown a patent distal bowel, intraoperative testing to confirm its patency was not performed. The resected colonic segment showed the presence of normal ganglion cells. The baby was mechanically ventilated for 48 hours, and feeding commenced on day 6 post-surgery. The baby was discharged on day 11 post-surgery at ad libitum.
Discussion
Colonic atresia (CA) is a congenital anomaly in which the colon is completely blocked or missing. The incidence is about 1 in 60,000 births. Since it is a rare condition, it is challenging for pediatric surgeons in terms of its diagnosis and management.[6] Disruption of intrauterine blood supply to the colon has been reported as the main cause of CA and usually happens at late gestation.[1], [2] The vascular abruption can be either attributed to intrinsic or extrinsic factors. The intrinsic factor may be any thrombo-embolic event originating from the placenta and passing through the fetal circulation to occlude the mesenteric vessels. As for the extrinsic factor, any possible intrauterine mechanical bowel obstruction that may compromise the blood supply can potentially cause CA such as intrauterine internal hernias, volvulus, or tight gastroschisis.[7]
CA is typically described to have 3 types; Type I, II, and III. Type I is described as the atretic segment with an intact bowel wall with luminal obstruction by a membrane (as in our case), and in Type II, the atretic blind ends are connected by a fibrous cord with intact mesentery. Type III is atresia with separated blind ends with a mesenteric gap. Type III CA is reported to be the most common type of CA proximal to the splenic flexure compared to Type I or II in distal CA.[1], [3]
The first survival of infants with CA was reported by Gaub in 1922. It was a case of sigmoid CA and managed by colostomy. The first primary repair was done by Potts in 1947 in transverse CA.[1], [3], [7], [8] Since then, many case series have been reported regarding various surgical repairs of CA with variable outcomes. However, none of them discussed specifically the side of the atresia, in which we believe that the different side carries its own unique features which require a different surgical approach. From the literature search, we tabulated 36 cases of right-sided CA (Table. 1). The majority of them were boys (n=23) and were diagnosed with type III CA (n=22). Out of these, twelve cases (33%) underwent primary anastomosis and three of them (25%) died, mostly from complications related to anastomotic leak or sepsis. Two out of 8 cases of the primary anastomosis with proximal diversion expired (25%), and only one diverted case expired (6.25%). In particular to the type of atresia, type I CA was present in 9 cases. Two of them had primary anastomosis, 4 had primary anastomosis with proximal diversion, while 3 were diverted.
It is clear from the table that most of the authors favored staged surgery rather than primary anastomosis and had better survival. The option of primary anastomosis has to be very selective and should be individualized based on the pathology and associated anomalies.[3] Isolated CA without any associated defects, as in our case, with good birth weight, is an example of a candidate for primary anastomosis.[8] In our case, we opted for primary anastomosis in view of the factors mentioned; along with the acceptable discrepancy after decompression and presence of incompetent ileocecal valve to ensure that no progressive dilatation of proximal colon is present after the surgery to compromise the anastomosis.[1], [3], [7], [9] Primary anastomosis carries a great advantage of having single surgery and single anesthesia, as supported by a series by Cox.[3] In cases where the primary colo-colic anastomosis is not favorable, a single staged repair can be achieved via resection of the proximal colon as reported by Gobran (2013) and Dassinger (2009) but it carries the morbidity of losing ileocecal valve such as malabsorption.[4]
Another important consideration for primary anastomosis in such cases is to deal with the bowel discrepancy. The primary anastomosis may be technically difficult with the presence of a huge discrepancy of proximal and distal bowel. Cox (2005) also suggested primary anastomosis to be done when the lumen discrepancy is less than 3:1 without distal bowel functional or mechanical obstruction. Few methods have been described in the literature for tackling this discrepancy, and Cheatle slit is one example. It is performed by making a small incision longitudinally on the antimesenteric border of the smaller caliber bowel to achieve a wider opening as close as the other larger bowel end. This will effectively alter the circumference of the small caliber bowel so that end-to-end anastomosis is possible.[10] Apart from this, end-to-oblique anastomosis is another method where the distal segment is resected at a 45-degree angle to increase the circumference size.[8] Besides that, another strategy is by making the larger caliber bowel smaller using the plication method or tapering.[10]
The normality of the distal colon also needs to be ensured before proceeding with primary anastomosis such as the presence of another distal colonic atresia or other anorectal anomalies, as well as the rare possibility of Hirschsprung disease. Careful perineal examination at birth is mandatory. Having a contrast enema done prior to the surgery will confirm the patency of the distal bowel; otherwise, a patency test needs to be done intraoperatively. Hirschsprung disease associated with CA has been reported.[4] In cases where Hirschsprung disease is a suspicion, it is recommended that frozen section should be done intraoperatively or otherwise to consider staged procedure by performing colostomy and subsequently to plan for rectal biopsy.[3], [11]
To conclude, primary anastomosis is feasible in cases of isolated right-sided colonic atresia, with no associated major comorbidity or defect, reasonable caliber for primary anastomosis, and a normal distal colon.
Notes
n1Conflicts of interest. Nil.
n3Author contributions: Author(s) declared to fulfill authorship criteria as devised by ICMJE and approved the final version. Authorship declaration form, submitted by the author(s), is available with the editorial office.
n4Consent to Publication: Author(s) declared taking informed written consent for the publication of clinical photographs/material (if any used), from the legal guardian of the patient with an understanding that every effort will be made to conceal the identity of the patient, however it cannot be guaranteed.
Acknowledgments
The authors would like to thank the Director-General of Health Malaysia for the permission to publish this paper.
References
|
|
| 1. |
Wester T. Colonic and rectal atresias. Newborn Surgery, Second Ed. 2003;457–64. |
| 2. |
El-Asmar KM, Abdel-Latif M, El-Kassaby A-HA, Soliman MH, El-Behery MM. Colonic atresia: Association with other Anomalies. J Neonatal Surg. 2016; 5:47. |
| 3. |
Cox SG, Numanoglu A, Millar AJW, Rode H. Colonic atresia: Spectrum of presentation and pitfalls in management. A review of 14 cases. Pediatr Surg Int. 2005; 21:813–8.
|
| 4. |
Dassinger M, Jackson R, Smith S. Management of colonic atresia with primary resection and anastomosis. Pediatr Surg Int. 2009; 25:579–82.
|
| 5. |
Gobran TA, Khalifa M, Kamal RMK. Different varieties of colonic atresia in a series of 13 patients: A single-center experience. Ann Pediatr Surg. 2013; 9:20–4.
|
| 6. |
Tripathy P, Jena P, Mohanty H. Clinical pattern of colonic atresia, management, and outcome in an Indian tertiary Care Center. J Clin Neonatol. 2020; 9:63.
|
| 7. |
Halder P, Kumar R, Mandal KC, Mondal G, Debnath B, Mukhopadhyay B, et al. A Clinico-pathological study of colo-rectal atresia. Pediatr Health Res. 2017; 2:1–6.
|
| 8. |
Montenegro Pinzon DA, Aragon Lopez SA, Valero Halaby JJ. Colonic atresia in a newborn. Case Report. Case Rep. 2018; 4:69–74.
|
| 9. |
Haxhija EQ, Schalamon J. Management of isolated and associated colonic atresia. Pediatr Surg Int. 2011; 27:411–6.
|
| 10. |
Frischer JS, Azizkhan RG. Jejunoileal Atresia and Stenosis [Internet]. 7th ed. Pediatric Surgery. Elsevier Inc.; 2012. 1059–1071 p. Available from: http://dx.doi.org/10.1016/B978-0-323-07255-7.00082-9.
|
| 11. |
Mansoor H, Kanwal N, Shaukat M. Atresia of the ascending colon: a rarity. APSP J Case Rep. 2010; 1:3.
|
| 12. |
Mirza B, Iqbal S, Ijaz L. Colonic atresia and stenosis: our experience. J Neonatal Surg. 2012; 1:4.
|
| 13. |
Saha H, Ghosh D, Ghosh T, Burman S, Saha K. Demographic study and management of colonic atresia: Single-center experience with review of the literature. J Indian Assoc Pediatr Surg. 2018; 23:206–11.
|
| 14. |
Hassan M. Type III wide colonic atresia: An etiological introspection. J Neonatal Surg. 2021; 10:39.
|