INTRODUCTION
Gastroschisis is characterized by the herniation of the midgut and occasionally other abdominal viscera through an anterior abdominal wall defect (AAWD), on the right of the umbilicus insertion, into the amniotic sac.[1], [2] There has been an increase in the incidence of gastroschisis from 2.5 to 4.4 per 10000 live births over the past few decades.[3] Several hypotheses of etiology have been put forward, but the recent dual vascular/thrombotic model better explained it.[4] Herein, we present two variants of vanishing gastroschisis in neonates detected postnatally, both being associated with jejunal atresia and extreme short bowel syndrome.
Case Series
Case 1:
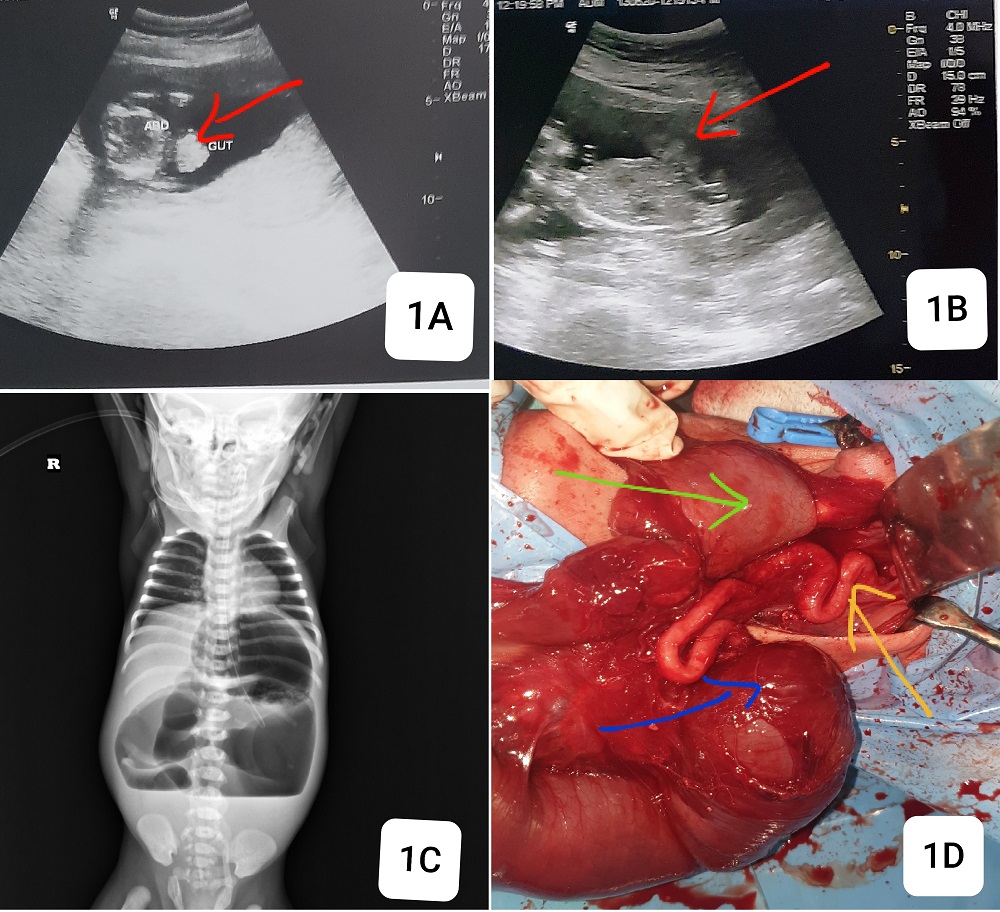
A 2-day-old female neonate was brought to our institute with bilious vomiting. On clinical examination, there was abdominal distension with visible palpable few loops, and a normal umbilicus. She was born to a 20-year-old primigravida. There was a history of antenatal diagnosis of gastroschisis. On analysis of her antenatal scans, the first obstetric scan at 16 weeks of gestation showed bowel herniation through an abdominal wall defect without any covering sac (Fig.1A,1B). Fetal anomaly scan at 20 weeks gestation was indifferent, but the 26-week scan showed additional findings of early-onset fetal growth restriction, normal amniotic fluid volume, and three vessels in the cord. Mother had labor pains at 32 weeks, and spontaneously delivered a female premature baby of 1.95kg at home. At birth, there was no visible abdominal wall defect, but the baby developed bilious vomiting and feeding intolerance on day one and referred to us by the pediatrician on day 2. Plain X-ray abdomen showed markedly dilated small bowel loops with air-fluid levels suggestive of obstruction (Fig.1C). The baby was resuscitated and optimized. On exploratory laparotomy, there was about 25 cm of remaining markedly dilated small bowel from gastro-duodenal junction to jejunal atresia (Type 1) and colon was about 15 cm. Most of the midgut structures including part of the jejunum, ileum, cecum, appendix, and most of the colon vanished (Fig.1D). A jejunostomy and distal colonic mucus fistula were formed after discussion of findings with the parents. On postoperative day 2, the stoma started functioning and feeding was started. After counseling, the parents choose palliative care at home. The patient died on the 25th day after surgery at home without any hospital visit.
Case 2:
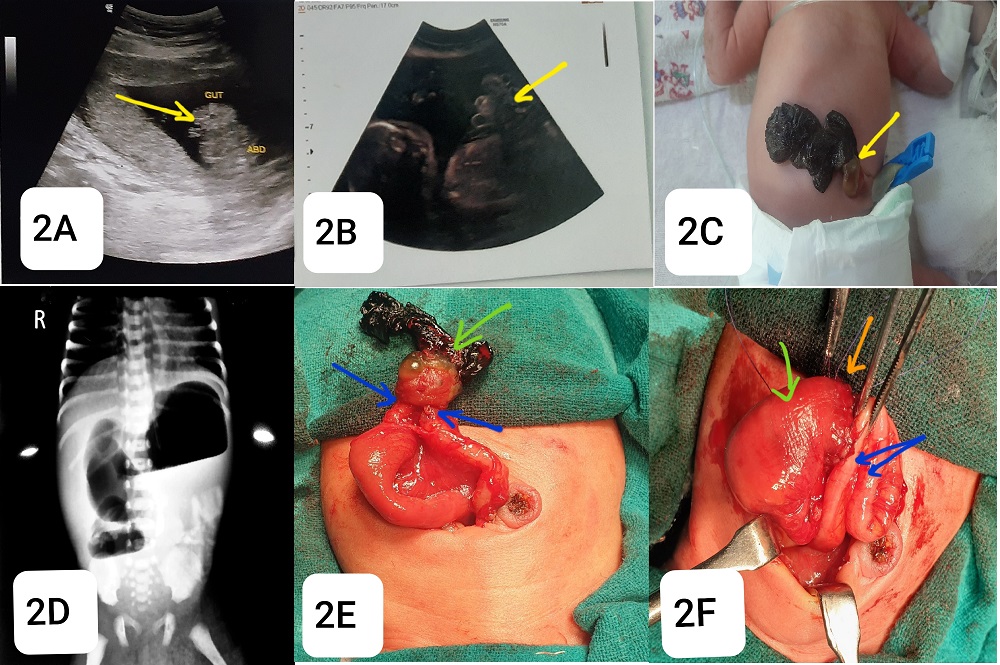
A 38-year-old primigravida mother delivered a female premature baby at a hospital setting by spontaneous vaginal delivery (weighing 1.5 kg at 33 weeks of gestation). After delivery, the baby was shifted to the Pediatric Surgical unit with bilious vomiting and a blackish mass visible at the central abdomen on day 1 of life. Antenatal scan at 18 weeks of gestation, showed bowel herniation through an abdominal wall defect without any covering sac (Fig.2A,2B). The karyotyping report was 46, XX. Antenatal scans were done on the 20th and 22nd weeks were indifferent. But a 26 weeks scan showed fetal growth restriction (FGR) below 1st centile with normal amniotic fluid volume, and three vessels in the cord. On clinical examination, there were visible gangrenous bowel loops protruding through a very small abdominal wall defect a cm right and above the umbilicus insertion with a gelatinous layer at the base (Fig 2C). Abdominal ultrasound and X-ray showed markedly dilated small bowel loops and very narrow abdominal wall defect of 7mm suggestive of obstruction with gastroschisis (Fig.2D). On exploratory laparotomy, there was dense adhesion between the peritoneum and protruding ends of the small bowel and colon at the narrow defect. The distal end of the small bowel and proximal end of the colon were atretic (Fig.2E). There was only about 20 cm remaining small bowel from the gastroduodenal junction to complete jejunal atresia and the colon was nearly 12 cm. Most of the midgut structures including part of jejunum, ileum, cecum, appendix, and most of the colon were absent (Fig.2F). A jejuno-colic end to back anastomosis was performed and the abdomen was closed in layers. Histology of the specimen revealed ischemic bowel necrosis. On postoperative day 4, the patient died of septicaemic shock.
Discussion
The hypotheses which could describe the finding of our patients are 1) Spontaneous partial or complete closure of anterior abdominal wall defect around the intestines and superior mesenteric artery, resulting in strangulation necrosis of the midgut; 2) incarceration and atrophy of protruding bowel at entry/exit with subsequent closure of the defect; 3) midgut volvulus at the narrow defect and resulting in gangrene.[5]
The incidence of vanishing gastroschisis is 4.5 to 6% of all gastroschisis.[6], [7] Regarding various possible phenotypic presentation of closing/closed gastroschisis, at one end, there is a closed abdominal ring with viable viscera; on the other extreme, it would be intestinal atresia at abdominal ring or infarction of midgut, intestinal resorption (matted thick fibrotic)m and normal-appearing abdominal wall termed as vanishing midgut.[6], [7] Kumar et al. classified phenotypically vanishing gastroschisis as Type I (vanishing gut with lumen), Type II (vanishing gut without lumen or nubbin of tissue), and Type III (antenatal evidence of gastroschisis and at the birth total absence of midgut).[8] Type III was the finding in case 1 neonate (closed VGS) whereas in case 2 it was of type II variety (Closing VGS).
In the present series, case 1 had spontaneous closure of antenatally diagnosed gastroschisis abdominal wall defect, whereas in case 2 there was closing gastroschisis with very narrow 7mm anterior abdominal wall defect, on the right side of the umbilicus. Spontaneous closure of gastroschisis defect in-utero was also observed by Barsoom et al.[9] Our cases had VGS with jejunal atresia and short atretic distal colon (extreme short bowel syndrome) which is extremely rare and rarely reported.[10], [11]
In neonates with gastroschisis, survival has approached up to 90% due to advancements in surgical techniques and neonatal care. But the prognosis of vanishing gastroschisis is still very poor due to short bowel syndrome. Mortality is due to short bowel syndrome, central-line related sepsis, and in the long-run total parenteral nutrition-related liver failure.[6], [7]
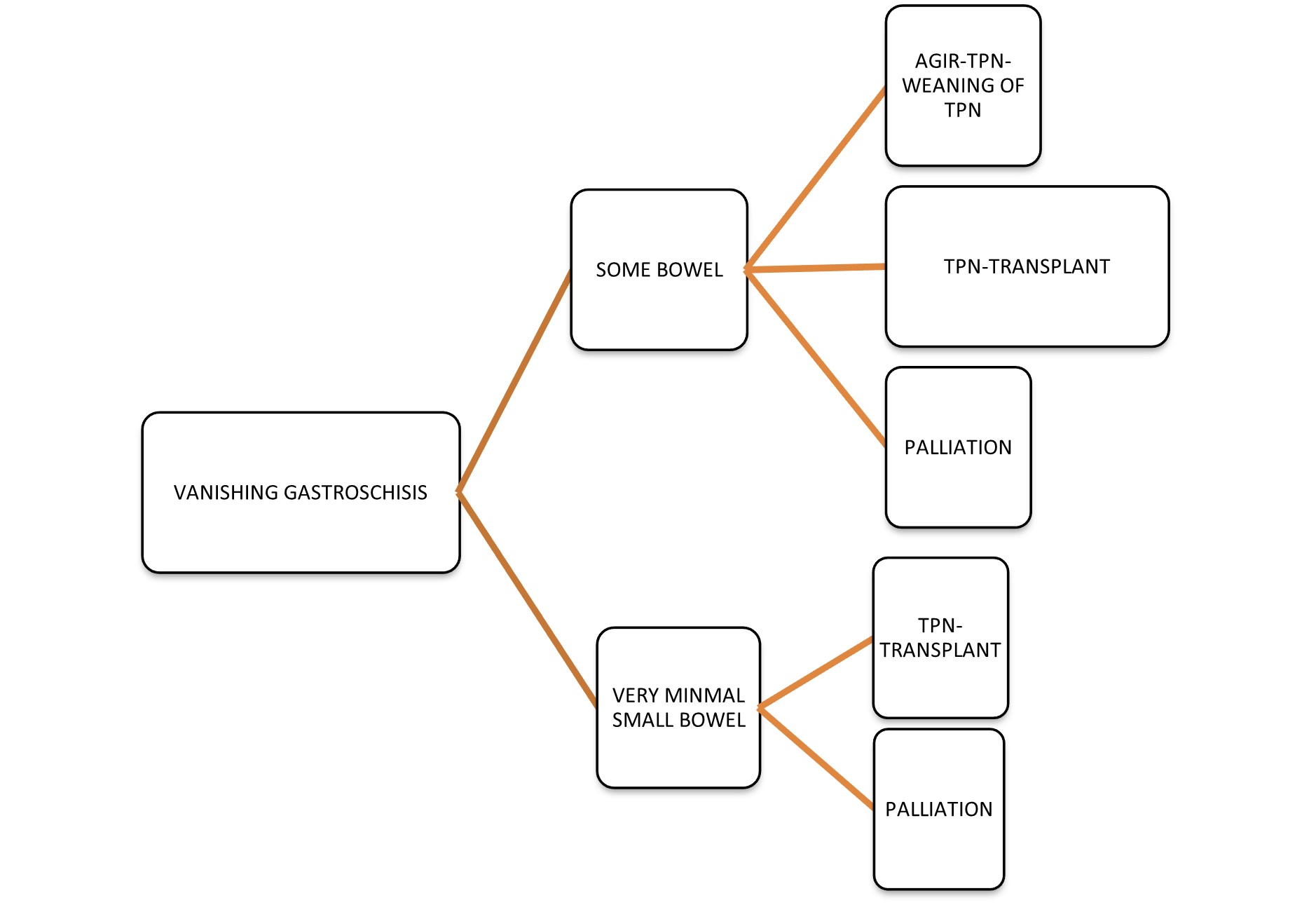
Vanishing gastroschisis usually needs an aggressive approach (Fig. 3). The only surgical options available for patients with some residual bowel are bowel lengthening procedures such as the Bianchi procedure, serial transverse enteroplasty procedure (STEP), and longitudinal intestinal lengthening and tapering (LILT). For patients with very minimal bowel, the only surgical option can be a small bowel transplant. All patients postoperatively require prolonged total parenteral nutrition (TPN), which can cause liver failure and may even require a liver transplant. Because of this, most of the parents usually choose palliative care plans and avoid aggressive surgical plans especially in developing countries.[11] Moreover, the outcomes of children after Bianchi surgery can be better when adequate bowel length is achieved. The 30 VGS survivors are summarized in (Table 1). In this series also the parents choose palliative procedures, jejunostomy in case 1 and end to back jejuno-colic anastomosis in case 2 & refused bowel lengthening procedures. In this series, both patients succumbed to their illness.
In conclusion, the prognosis of complicated vanishing gastroschisis is still very poor with high morbidity and mortality. In-utero vascular accidents in gastroschisis lead to jejunal atresia or short bowel syndrome. Advancements in neonatal bowel transplants in the future may improve the survival of patients with short bowel syndrome.
Notes
AGIR: autologous gastrointestinal reconstruction, GA: gestational age (weeks), BW: birth weight (KG), SBL: small bowel length distal to the duodenojejunal junction(cm), STEP: serial transverse enteroplasty Procedure, LILT: longitudinal intestinal lengthening and tapering, NS: not specified, W: weeks, M: months, Y: years.
n1Conflicts of interest. None
n3Author contributions: Author(s) declared to fulfill authorship criteria as devised by ICMJE and approved the final version. Authorship declaration form, submitted by the author(s), is available with the editorial office.
n4Consent to Publication: Author(s) declared taking informed written consent for the publication of clinical photographs/material (if any used), from the legal guardian of the patient with an understanding that every effort will be made to conceal the identity of the patient, however it cannot be guaranteed.
References
|
|
| 1. |
Frolov P, Alali J, Klein MD. Clinical risk factors for gastroschisis and omphalocele in humans: a review of the literature. Pediatr Surg Int. 2010; 26:1135-48 |
| 2. |
Schwartz MZ, Timmapuri SJ. Gastroschisis. In: Spitz L, Coran AG, eds. Operative pediatric surgery. 7th ed. CRC press: Taylor & Francis group; 2013; 309-19. |
| 3. |
Kilby MD. The incidence of gastroschisis. BMJ. 2006; 332:250-1.
|
| 4. |
Sergi C, Hager T, Alge A, Hager J. Vanishing gastroschisis: Good outcome after a 10-year follow-up. J Pediatr Surg Case Rep. 2018; 30:77–81.
|
| 5. |
Basaran UN, Inan M, Gücer F, Yardim T, Pul M. Prenatally closed gastroschisis with midgut atresia. Pediatr Surg Int. 2002; 18:550-2.
|
| 6. |
Vogler SA, Fenton SJ, Scaife ER, Book LS, Jackson D, Nichol PF, et al. Closed gastroschisis: total parenteral nutrition–free survival with aggressive attempts at bowel preservation and intestinal adaptation. J Pediatr Surg. 2008; 43:1006–10.
|
| 7. |
Houben C, Davenport M, Ade Ajayi N, Flack N, Patel S. Closing gastroschisis: diagnosis, management, and outcomes. J Pediatr Surg. 2009; 44:343-7.
|
| 8. |
Kumar T, Vaughan R, Polak M. A proposed classification for the spectrum of vanishing gastroschisis. Eur J Pediatr Surg. 2013; 23:72-5.
|
| 9. |
Barsoom MJ, Prabulos A, Rodis JF, Turner GW. Vanishing gastroschisis and short-bowel syndrome. Obstet Gynecol. 2000; 96:818-9.
|
| 10. |
Sisodiya RS, Panda SS, Gupta CK, Sinha SK. Closed gastroschisis with vanished small bowel and jejunal atresia. J Neonatal Surg. 2016; 5:65.
|
| 11. |
Dennison FA. Closed gastroschisis, vanishing midgut, and extreme short bowel syndrome: Case report and review of the literature. Ultrasound. 2016; 24:170-4.
|
| 12. |
Kimble RM, Blakelock R, Cass D. Vanishing gut in infants with gastroschisis. Pediatr Surg Int. 1999; 15:483–5.
|
| 13. |
Ogunyemi D. Gastroschisis complicated by midgut atresia, absorption of bowel, and closure of the abdominal wall defect. Fetal Diagn Ther. 2001; 16:227–30.
|
| 14. |
Winter LW, Giuseppetti M, Breuer CK. A case report of midgut atresia and spontaneous closure of gastroschisis. Pediatr Surg Int. 2005; 21:415–6.
|
| 15. |
Sandy JE, Lazar LF, Helm s RA. Vanishing bowel: A therapeutic challenge. Nutr Clin Pract. 2006; 21:401–7.
|
| 16. |
Buluggiu A, Haddad M, Coste M, Louis-Borrione C, Ughetto F, Guys JM, et al. Intestinal loop lengthening: early treatment of vanishing bowel. Pediatr Surg Int. 2009; 25:449.
|
| 17. |
Khalil BA, Gillham JC, Foresythe L, Harding R, Johnston T, Wright C, et al. Successful management of short gut due to vanishing gastroschisis–case report and review of the literature. Ann R Coll Surg Engl. 2010; 92:e10–3.
|
| 18. |
Lawther S, Philip I. The outcome of closing gastroschisis: two case reports and literature review. Eur J Pediatr Surg. 2010; 20:65-6.
|
| 19. |
Dahl E, Haugen G, Refsum S. Midgut atresia and spontaneously closed gastroschisis: support for a mechanical explanation. Eur J Pediatr Surg. 2011; 21:128–30.
|
| 20. |
Wood SJ, Samangaya RA, Gillham JC, Morabito A. Gastroschisis and the risk of short bowel syndrome: outcomes and counselling. Neonatol. 2014; 105:5-8.
|
| 21. |
Abdel-Latif M, Soliman MH, El-Asmar KM, Abdel-Satta r M, Abdelraheem IM, El-shafei E. Closed gastroschisis. J Neonatal Surg. 2017; 6:61.
|
| 22. |
Singh JK, Yadav DK, Khanna K, Khanna V. Closed gastroschisis with left defect: a rare variant. BMJ Case Rep. 2018: bcr-2017.
|
| 23. |
Ponce MM, Hermans D, Magnee C, Hubinont C, Biard JM. Vanishing gastroschisis visualized by antenatal ultrasound: a case report and review of literature. Eur J Obstet Gynecol Reprod Biol. 2018; 228:186–90.
|
| 24. |
Rached EA, Sananes N, Kauffmann-Chevalier I, Becmeur F. Vanishing gastroschisis with a favorable outcome after a 3-year follow-up: A case report and Literature review. Case Rep Obstet Gynecol. 2020; 2020:1-6.
|