Figures
|
|
|
Figure 1
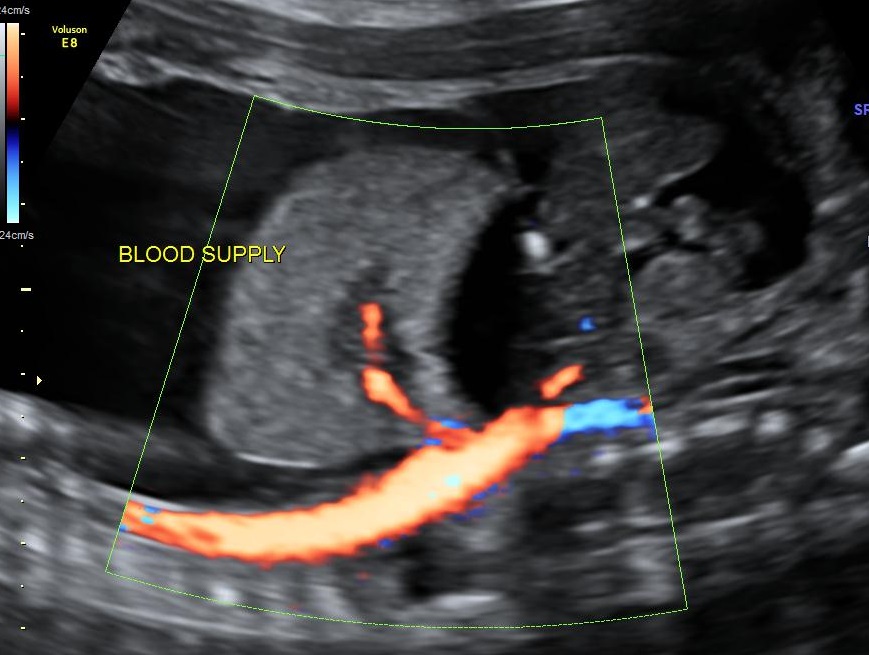
Ultrasound image of BPS with severe NIH. |
|
|
|
|
Figure 2
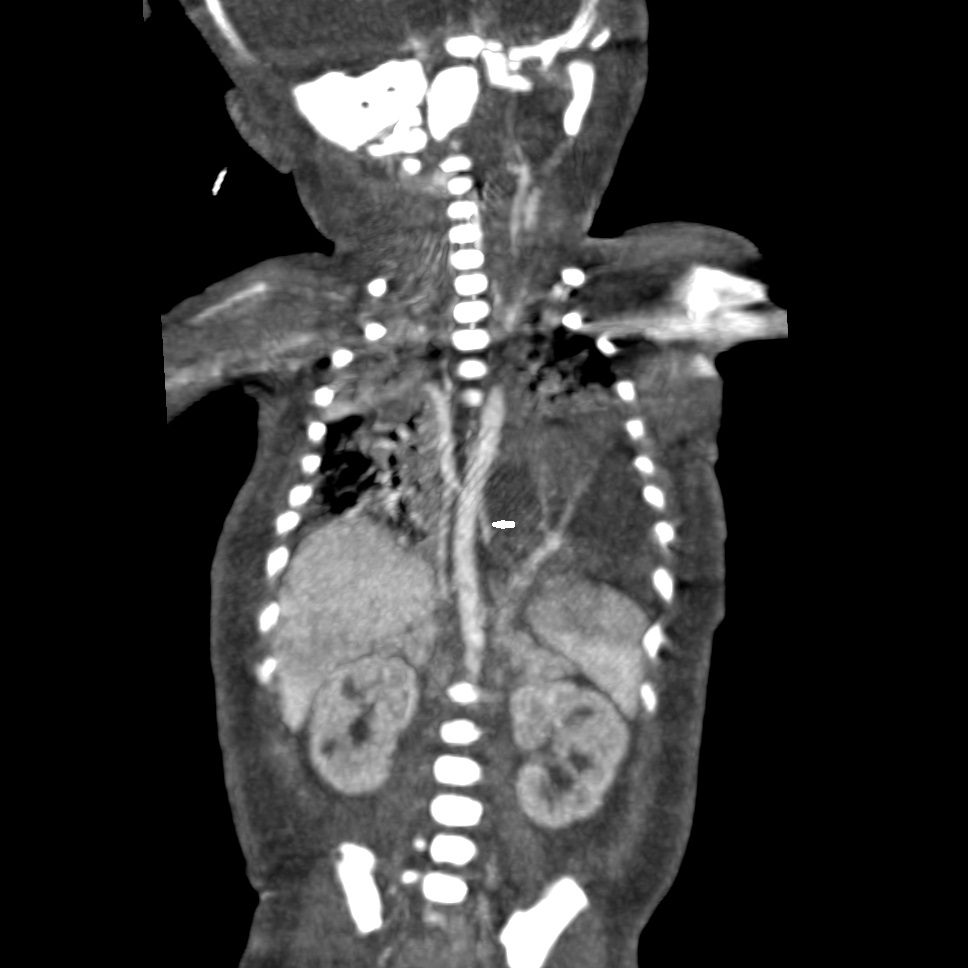
CT thorax showing the feeding vessel to the ELS from the aorta. |
|
|
|
|
Figure 3
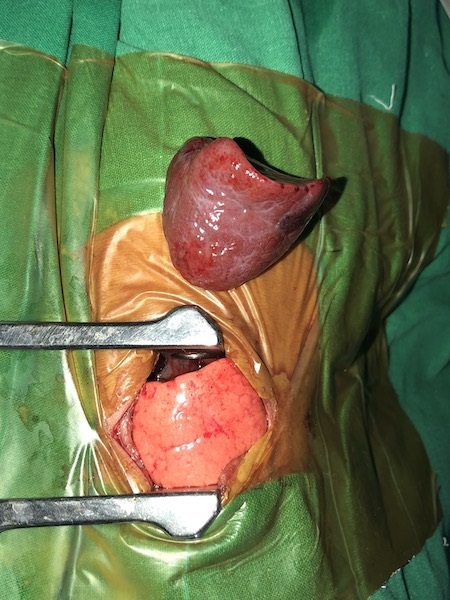
Excised BPS. |
|
|
© 2021, Mandke et al
Received Day: 28 Month: 07 Year: 2020 Accepted Day: 07 Month: 12 Year: 2020 J Neonatal Surg. 2021; 10: 5. DOI: 10.47338/jns.v10.556 |
|
Keywords: Bronchopulmonary, Extralobar, Sequestration, Hydrops, Thoracocentesis. |
||
Bronchopulmonary sequestration (BPS) is a congenital lung anomaly that consists of a discrete mass of lung tissue that does not communicate with the tracheobronchial tree and receives its blood supply from the systemic circulation. Of these, 95% are intrathoracic and 5% are extrathoracic. Intrathoracic BPS are classified into 2 types, intralobar sequestrations (75%) and extralobar sequestrations (ELS) (25%).[1]
Most BPS regress in-utero, though a large BPS may be associated with mediastinal shift causing complications like polyhydramnios, cardiac failure, and NIH.[2] NIH associated with BPS is quite rare (6.8-10%); and if untreated in the fetal period, have a highly unfavorable outcome, with a 95% risk of intrauterine fetal demise.[3], [4] We herein report a case of NIH complicating BPS that was managed successfully.
A 36-year-old lady, (G3P1L1A1) 27+6 weeks gestation, presented to our fetal medicine department with an ultrasound study showing a hyperechoic intrathoracic mass in the fetal left hemithorax measuring 3.8x3.1x3.5cms, which was separate from the fetal lung with a large vessel arising from the aorta perfusing it (Fig.1). There was gross left-sided pleural effusion with mediastinal shift causing pseudo-dextrocardia and ascites with hydrops. A diagnosis of BPS with severe NIH was made. There was significant blood flow across the tricuspid valve and descending thoracic aorta, which implied that the fetal hydrops was due to high-output cardiac failure secondary to a ‘diastolic run-off’ from the aorta.
We planned for immediate thoracocentesis and a possible need for thoracoamniotic shunt. Under ultrasound guidance, 90ml of pleural fluid was tapped which was sent for cytology. On the following day, the ultrasound showed refilling of the left-sided pleural effusion, worsening hydrops, and progressive cardiac failure, which prompted a decision for an emergency cesarean section rather than further fetal interventions. Repeat pleural tapping just before delivery was done to improve neonatal resuscitative measures. The baby required resuscitation and intubation in the delivery room and positive pressure ventilation was instituted. A left-sided intercostal drain (ICD) was inserted immediately at birth. After tube thoracocentesis, the weight of the baby was recorded as 1 kg.
Postnatal CT of the chest reported a 6x3.6x3.3cm ELS in the left lower hemithorax with arterial supply from descending thoracic aorta and venous drainage into the hemiazygos vein (Fig.2). Postnatal management involved skillful ventilation and cardiac stabilization. After reasonable stabilization, the baby underwent sequestrectomy by a conventional left thoracotomy on Day 8 of life (Fig.3). Histologically the mass was confirmed to be BPS.
The baby developed a contralateral pleural effusion on the 2nd postoperative day, which required drainage with a right-sided intercostal drain. The drain output gradually reduced over 7-10 days after which both the ICDs were removed and the baby was discharged in good condition. The baby is doing fine on the follow-up of 6 months.
The first case of BPS associated with NIH was published by Romero et al. in 1981.[5] The prognosis of fetal BPS appears to rely on the presence or absence of pleural effusion and NIH. The reason behind pleural effusion associated with BPS is postulated to be a result of the torsion of the narrow vascular pedicle of the sequestrated lobe, obstructing the efferent lymphatic channels and veins.[6] On the other hand, the development of NIH is most probably secondary to high-output cardiac failure due to vascular steal which may occur in the presence of an anomalous systemic artery and venous drainage via pulmonary or systemic veins.[6]
In cases of BPS without effusion or NIH, expectant management is followed, since the probability of regression is much higher.[7] Since the chances of spontaneous regression in cases of BPS with hydrops are very low [3], [7], these are the fetuses, which require targeted intervention to improve their outcome. The outcome of babies with BPS with NIH has been dismal, until the advent of fetal interventions in the 1990s. Fetal therapeutic choices include non-invasive fetal interventions like inotropic therapy, antenatal steroids, or therapy with digoxin and furosemide, minimally invasive fetal interventions like fetal thoracentesis, thoracoamniotic shunt, radiofrequency ablation/ laser coagulation/ thrombogenic coil embolization of feeding vessel, and fetal surgery.
An extensive review of literature disclosed less than 80 published cases of BPS developing NIH which have survived after fetal therapy worldwide. [4], [8], [9] Cavaretto et al., in 2008 reviewed (in addition to their 8 cases) 31 cases of ELS with NIH published till 2008; these cases were treated with minimally invasive fetal therapy, and only 2 postnatal deaths were recorded confirming that fetal intervention in BPS with NIH positively improves the prognosis.[9] In 2018, in a landmark paper, Cruz-Martinez et al. reported full laser ablation of the feeding artery (FLAFA), successfully performed in 15 cases of BPS with pleural effusion/NIH.[8] They found that FLAFA causes the disappearance of all fetal effusions, regression of the sequestration, and normal pulmonary growth. In another longitudinal study in 2018, Riley et al. reviewed 103 fetuses of BPS, four of that developed hydrops which were given maternal betamethasone; three fetuses underwent thoracentesis and/or thoracoamniotic shunt placement with subsequent hydrops resolution. All fetuses survived.[3]
To date, in the event of unavailability or failure of fetal interventions, successful postnatal outcomes are still few. In our case, we would have preferred to put in a thoracoamniotic shunt, but because of the rapidly increasing hydrops and cardiac dysfunction, it was not possible, and instead, it necessitated early delivery. An early sequestrectomy was done since the artery supplying the BPS was quite large and optimization was difficult in the face of impending cardiac failure. A minimally invasive technique was not possible because of hemodynamic instability.
To conclude, BPS with NIH is rare and has a dismal prognosis without fetal intervention. This case is the smallest baby of BPS with NIH successfully treated postnatally.

|
|
|
Figure 1
Ultrasound image of BPS with severe NIH. |
|

|
|
|
Figure 2
CT thorax showing the feeding vessel to the ELS from the aorta. |
|

|
|
|
Figure 3
Excised BPS. |
|
n1Conflicts of interest. The authors declare that they have no conflict of interest
n2Source of Support: Nil
n3Author contributions: Author(s) declared to fulfill authorship criteria as devised by ICMJE and approved the final version. Authorship declaration form, submitted by the author(s), is available with the editorial office.
n4Consent to Publication: Author(s) declared taking informed written consent for the publication of clinical photographs/material (if any used), from the legal guardian of the patient with an understanding that every effort will be made to conceal the identity of the patient, however it cannot be guaranteed.
None
| 1. | El Mhabrech Houda ZA, Amine K, Amina BS, Raja F, Chiraz H. Antenatal diagnosis of extralobar pulmonary sequestration. Pan Afr Med J. 2014; 19:54. |
| 2. | Zhang H, Tian J, Chen Z, Ma X, Yu G, Zhang J, et al. Retrospective study of prenatal diagnosed pulmonary sequestration. Pediatr Surg Int. 2014; 30:47-53. |
| 3. | Riley JS, Urwin JW, Oliver ER, Coleman BG, Khalek N, Moldenhauer JS, et al. Prenatal growth characteristics and pre/postnatal management of bronchopulmonary sequestrations. J Pediatr Surg. 2018; 53:265-9. |
| 4. | Pock R, Straňák Z, Vojtěch J, Hašlík L, Feyereisl J, Krofta L. Bronchopulmonary sequestration with fetal hydrops in a monochorionic twin successfully treated with multiple courses of betamethasone. AJP Rep. 2018; 8:e359. |
| 5. | Romero R, Chervenak FA, Kotzen J, Berkowitz RL, Hobbins JC. Antenatal sonographic findings of extralobar pulmonary sequestration. J Ultrasound Med. 1982; 1:131-2. |
| 6. | Upadhyay A, Aggarwal R, Choudhry S. Hydrops fetalis and extralobar lung sequestration. Indian Pediatr. 2002; 39:392-5. |
| 7. | Mallmann MR, Geipel A, Bludau M, Matil K, Gottschalk I, Hoopmann M, et al. Bronchopulmonary sequestration with massive pleural effusion: pleuroamniotic shunting vs intrafetal vascular laser ablation. Ultrasound Obstet Gynecol. 2014; 44:441-6. |
| 8. | Cruz-Martínez R, Nieto-Castro B, Martínez-Rodríguez M, Gámez-Varela A, Ahumada-Angulo E, Luna-García J, et al. Thoracic changes after full laser ablation of the feeding artery in fetuses with bronchopulmonary sequestration. Fetal Diagn Ther. 2018; 44:166-72. |
| 9. | Cavoretto P, Molina F, Poggi S, Davenport M, Nicolaides KH. Prenatal diagnosis and outcome of echogenic fetal lung lesions. Ultrasound Obstet Gynecol. 2008; 32:769–83. |