Introduction
Pneumothorax is the most common cause of air leak. It occurs when the air trapped in the alveoli moves through the parietal pleura into the pleural space, equilibrating pressures and eventually collapsing a segment of the lung.[1] A persistent air leak (PAL) is one that lasts for more than 5-days. It is associated with an increase in morbidity and hospitalization costs. [2], [3], [4]
Autologous blood patch pleurodesis (ABPP) has been used in adults as an efficient and safe technique. [3], [5], [6] A fibrin glue patch is also an alternative, but its elevated cost and technique can limit its routine use. Evidence of their use in the pediatric population, specifically in the neonatal age is scant.
Autologous blood patch pleurodesis (ABPP) technique
Blood is drawn from the patient (2cc/kg from peripheral access), and administered into the pleural cavity through the chest tube. A 10 cc 0.9% NaCl flush is then used to deliver the blood completely into the pleural cavity. The chest tube collecting device is placed at least 60 cm above the patient’s headrest for 30 min. (Allowing air drainage and avoiding blood return). The device is placed on a water seal, negative pressure is not used.[3]
Case Series
Case 1:
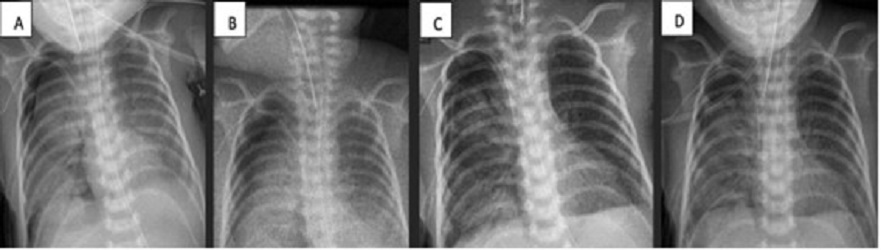
A 9-day-old female baby, born at 32 GW (1224 g), diagnosed with early onset sepsis, respiratory distress syndrome, and pulmonary hypertension. Placed on HFV with FiO2 at 94%. Due to right tension pneumothorax, chest tube placement was also required. Pneumothorax persisted for more than 5 days (labeled when air bubbles continue to drain nito underwater seal device for more than 5 days). ABPP was performed and improvement was noted at 48 hours (no bubbles, radiographic improvement, and decrease in ventilatory parameters). The chest tube was removed on the 19th day of life (Fig. 1).
Case 2:
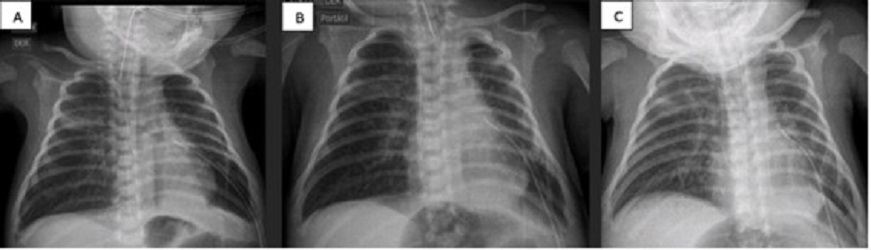
A 16-day-old male baby, born at 39 GW (3500 g) (S/P left diaphragmatic hernia repair and chest tube removal at day 15), developed left-sided tension pneumothorax. It was managed with ventilatory support on SIMV with FiO2 at 35%, and chest tube placement. PAL was encountered for more than 7 days. ABPP was performed and improvement was noted at 24 hours (no bubbles, radiographic improvement, and decrease in ventilatory parameters). The chest tube was removed on the 19th day of life (Fig. 2)
Case 3:
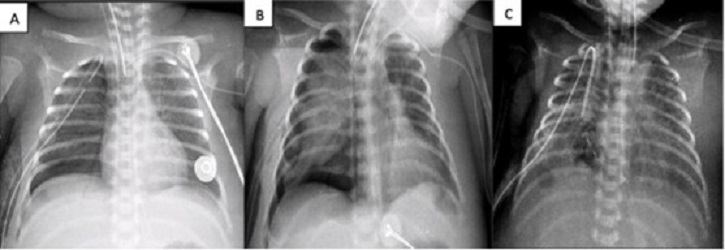
A 17-day-old male, born at 29 GW (1200 g), was diagnosed with NEC, respiratory distress syndrome, and pulmonary hypertension. (NEC required ex-laparotomy with Santully type ileostomy). Placed on CMV with FiO2 at 81% due to right tension pneumothorax. Air leakage persisted for 4 days along with increasing ventilatory parameters, that warranted ABPP (though the PAL criterion was not met fully). On day 23, ABPP was re-performed. No clinical improvement was seen. The patient developed hemodynamic instability requiring emergent thoracotomy. Multiple lesions were seen in the inferior lobe of the right lung. Primary closure was done without success, therefore fibrin patch (bovine serum purified albumin glutaraldehyde) was placed. Improvement was evident at 24 hours. The chest tube was removed on the 33rd day of life (Fig. 3).
Discussion
The exact mechanism of the ABPP in pneumothorax is not established. Possible mechanisms of action are coagulation of blood which might seal the air leak and inflammatory reaction such as the one created by mechanical or chemical pleurodesis. Most likely it is a combination of both mechanisms. [7], [8], [9]
We found only one case report of ABPP in neonates. Huseynov et al. [6] presented a case of PAL of 17 days duration on a neonate with a chest tube in place. This patient required 3 sessions of ABPP; clinical improvement was evident after 48 hours of the 3rd patch. Lillegard et al. [3], published a case series in pediatric patients (youngest being 2 month-old) where ABPP worked successfully, except in one case (pneumothorax recurred). Tatewaki et al. [10] reported a case of a 3-month-old infant with hypoplastic left heart syndrome who developed bilateral pneumothoraces, treated successfully with ABPP.
In our series, all the 3 cases had a satisfactory outcome, as shown by the resolution of pneumothorax (irrespective of etiology). The technique has been previously described. We recommend postural changes to allow a better redistribution of the blood patch. [3] No complications or increase in morbidity were seen with this procedure.
ABPP is broadly used in the pediatric population and its use in neonates is feasible and promising. It is considered a minimally invasive procedure, with an easily replicated technique. It can be done at the bedside and has shown to be cost-effective. Nevertheless, long-term studies are needed to determine the efficacy and safety of ABPP.
Regarding our cases, 2 are still being followed up in the outpatient clinic without any signs or symptoms of respiratory compromise. The patient on which the fibrin patch was placed died due to other comorbidities (not related to his respiratory status).
Conculusion
The use of ABPP as an alternative for the treatment of neonates with PAL is a safe and efficient technique. The procedure is replicable and can be done at the bedside at a low cost. A fibrin glue patch should be considered as an alternative option when ABPP fails to resolve the leak. Further studies are required to determine the safety and efficacy of ABPP, as well as compare it with other treatment methods.
Notes
n1Conflicts of interest. None.
n3Author contributions: Author(s) declared to fulfill authorship criteria as devised by ICMJE and approved the final version. Authorship declaration form, submitted by the author(s), is available with the editorial office.
n4Consent to Publication: Author(s) declared taking informed written consent for the publication of clinical photographs/material (if any used), from the legal guardian of the patient with an understanding that every effort will be made to conceal the identity of the patient, however it cannot be guaranteed.
References
|
|
| 1. |
Sahn S, Heffner J. Spontaneous pneumothorax. N Engl J Med. 2000; 342:868–74. |
| 2. |
Cerfolio RJ, Bryant AS. The management of chest tubes after pulmonary resection. Thoracic Surgery Clinics. 2010; 20:399-405. |
| 3. |
Lillegard JB, Kennedy RD, Ishitani MB, Zarroug AE, Feltis B. Autologous blood patch for persistent air leak in children. J Pediatr Surg. 2013; 48:1862-6.
|
| 4. |
Lackey A, Mitchell JD. The cost of air leak: Physicians’ and Patients’ perspectives. Thor Surg Clin. 2010; 20:407-11.
|
| 5. |
Cobanoglu U, Melek M, Edirne Y. Autologous blood pleurodesis: A good choice in patients with persistent air leak. Ann Thorac Med. 2009; 4:182–6.
|
| 6. |
Huseynov M. A first case report of neonatal persistent pneumothorax treated with an autologous blood patch. Turkish Arch Pediatr. 2020; 55:438–40.
|
| 7. |
Rinaldi S, Felton T, Bentley A. Blood pleurodesis for the medical management of pneumothorax. Thorax. 2009; 64:258–60.
|
| 8. |
Dumire R, Crabbe MM, Mappin FG, Fontenelle LJ. Autologous “blood patch” pleurodesis for persistent pulmonary air leak. Chest. 1992; 101:64–6.
|
| 9. |
Manley K, Coonar A, Wells F, Scarci M. Blood patch for persistent air leak: A review of the current literature. Opin Pulm Med. 2012; 18:333–8.
|
| 10. |
Tatewaki H, Nakano T, Hinokiyama K, Ebuoka N, Matsumae H, Machida D. Successful treatment for prolonged air leaks with an autologous “blood patch” pleurodesis after the Norwood procedure. Jpn J Cardiovasc Surg. 2014; 43:340–3.
|