Introduction
Gastroschisis is a type of congenital paraumbilical, full-thickness anterior abdominal wall defect on the right side of the umbilical ring with uncovered abdominal contents (usually small and large intestines) protruding out through the defect.[1], [2] It has an incidence of 1 in 4,000 live births.[1], [2] Malformations involving other major organ systems are uncommonly associated with gastroschisis, and they are usually associated with either infarction or atresia of the prolapsed bowel.[3], [4] Reduction of the abdominal contents is crucial within hours after birth as the neonate is at risk for water and heat loss from the exposed bowel, compromised intestinal circulation, and infection.[4]
Several factors like (i) antenatal diagnosis, (ii) mode of delivery, (iii) type of abdominal wall closure, (iv) associated malformations, (v) intestinal atresia, and (vi) NEC have a bearing on the final outcome of neonates with gastroschisis.[2], [5] Gastroschisis is often diagnosed antenatally in developed nations. As per the literature, the survival rate of neonates with gastroschisis is over 90%.[6] This is because of the developments in prenatal care, and neonatal intensive care in the developed nations.[6] In contrast, these defects are encountered postnatally at most of the primary healthcare centers in India and then referred to tertiary institutes. The morbidity and mortality in our geographical area are under-evaluated. We did this study with the aim of studying the early outcomes of Gastroschisis from a tertiary care institute in India.
Methods
A single-center, prospective, observational study was conducted in the Department of pediatric surgery of SMS Medical College, Jaipur for one year extending from January to December 2021. Ethical clearance (IEC No. 548/MC/EC/2021) was obtained from the institutional ethical committee before the initiation of the study.
Case definition: Gastroschisis was defined as a congenital malformation characterized by visceral herniation through a paraumbilical, full-thickness abdominal wall defect on the right side of the umbilical ring with an intact umbilical cord on the left side.[7]
Inclusion criteria: All patients with Gastroschisis presenting in the neonatal period.
Exclusion criteria: Neonates with Gastroschisis presenting postoperatively from other centers.
Methodology: Informed written consent was obtained for the study. Clinical evaluation of all the patients was performed including a detailed ante-natal and post-natal history, complete clinical examination, and local examination of the lesion(s) and co-morbidities. The neonates were assessed concerning their demographic characteristics (age, sex, weight, prematurity, antenatal diagnosis, etc.), and other associated anomalies. Their vitals, hydration status, eviscerated intestinal loops and viscera, anterior abdominal wall, and fecal discharge were recorded. The presence of cardiac murmur, condition of the spine, and inguinoscrotal region were observed.
Management of new-born was initiated with resuscitation and stabilization in the surgical neonatal intensive care unit (NICU). Attention was given to maintaining a warm environment, keeping the baby dry, and preventing heat loss. The herniated visceral contents were enclosed with warm saline-soaked sterile gauze and bandages. The umbilical clamp was replaced with a cord tie using sterilized thread. Blood glucose levels were tested in neonates. Nasogastric decompression and bladder catheterization were performed. Intravenous access was accomplished in the upper extremities for fluid resuscitation and broad-spectrum prophylactic antibiotics. Bianchi reduction without anesthesia was considered after stabilization in the newborns who were deemed suitable according to their general condition, the size of the defect, herniated organs, and the presence of viscero-abdominal disproportion (VAD).[8] The surgical procedure (primary closure or silo application) for a patient was decided according to the clinical condition of the neonate, eviscerated bowel, and the preferences of the operative team. Close monitoring of respiratory status was done for the first 72 hours postoperatively because of the increased intra-abdominal pressure (IAP) and its adverse hemodynamic consequences. Total parenteral nutrition (TPN) was initiated in all neonates, 24 to 48 hours postoperatively. A minimum of seven to ten days of bowel rest, supportive care, and vigilant clinical-radiographic monitoring were performed.
Statistical evaluation: All the medical records were carefully recorded in the Excel sheets and charts were prepared. Statistical analysis was done by using a statistical package for social sciences (SPSS software version 20, IBM, India).
Results
There were 30 male and 28 female patients with M:F Ratio of 1.07:1 (Table. 1). There were 37 (63.79%) preterm (premature) and 21 (36.21%) term neonates (Table. 2) . None of the patients were diagnosed antenatally. All the patients were referred to our center from other hospitals without proper protection of the eviscerated bowel. The birth weight ranged between 1300g and 3000g and the mean value was 2019±357g (Table. 1) Four (6.89%) patients were very low birth weights (<1500g); none of these patients survived.
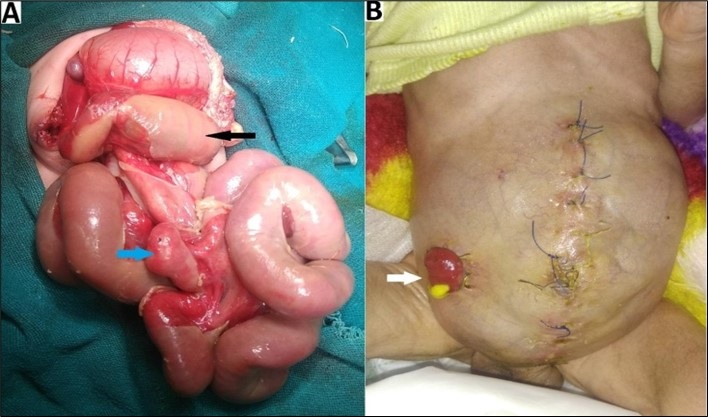
Seven patients (12.07%) had major additional anomalies. Four (6.89%) were diagnosed with cardiac anomaly (clinical/ECHO) and two (3.44%) had intestinal atresia. One (1.72%) patient had a lumbosacral meningomyelocele. Undescended testes (unilateral/bilateral) were present in 11/30 (36.67%) male patients. One of the patients with intestinal atresia was managed with a stoma (ileostomy) made during primary skin flap closure (Fig. 1), while the other patient was primarily closed. The latter patient died before a second-look laparotomy could be performed to repair atresia. A syndromic evaluation was not contemplated in our study.
Three neonates did not have any operative procedures. When herniated organs were carefully reviewed, the small intestines were herniated outside the body in 100%, the colon in 87.93% (51), and the stomach in 72.41% (42) of the patients. Bianchi reduction could not be attempted in any patient due to the poor general condition of the neonate and the quality of the eviscerated bowel. Fifty (86.21%) patients underwent primary skin flap closure, while staged reduction with silo was performed in five (8.62%) patients. All five patients with staged silo reduction were attempted initially with primary skin flap closure. The second operation could not be performed on any patient with a silo because of postoperative mortality (within one to seven days). The second operation (resuturing the wound margins for the exposed bowel loop) was performed on one (1.72%) patient with skin flap closure. TPN was initiated in all the neonates until they were able to tolerate oral intake.
Among 55 (94.83%) patients with surgical procedures, 26 (47.27%) could be salvaged. Out of a total of 58 cases, favorable (survival) outcomes were seen in 26 (44.83%) patients. Thirty-two (55.17%) patients had unfavorable (mortality) outcomes. Mortality was high (92.59%, 25/27) in the patients presenting with markedly edematous bowel with leathery peel. Mortality was higher (59.46%, 25/37) in preterm (premature) neonates than in term neonates (47.62%, 10/21) [Table 2]. There was no statistically significant difference in mortality between the genders.
The causes of death in three patients who died before any therapeutic procedure were late presentation (>48 hours of birth), sepsis, and hypothermia. Seventeen patients (29.31%, 17/58) succumbed to death in the first 72 hours with abdominal compartment syndrome (ACS) due to VAD, followed by acute renal failure, acidosis, and finally multi-organ failure in the early period. All these patients underwent primary skin flap closure. Eight patients (13.79%, 8/58) died due to postoperative sepsis with thrombocytopenia. Intestinal perforation with (or without) NEC developed in two (3.44%, 2/58) patients. In two (3.44%, 2/58) patients the cause of death could not be confirmed. The duration of hospital stay in neonates who survived ranged from one to 4 weeks. The overall hospital stay ranged from one day to four weeks.
Discussion
Abdominal wall defects are a complex group of congenital abnormalities with a broad spectrum of manifestations and include mainly omphalocele, gastroschisis, hernia of the umbilical cord, Exstrophy bladder, and rarely patent Vitello-intestinal duct.[1] Gastroschisis is a common defect and is the focus of this study. Gastroschisis is characterized by the complete absence of the abdominal wall usually to the right of the umbilical cord with evisceration of abdominal contents (lacks peritoneal covering) which include small and large intestines, sometimes stomach and fallopian tubes (females) and occasionally liver and spleen.[3], [9], [10] The defect is usually between 2 to 5 cm in size, with a normally developed and placed umbilicus. It has a prevalence of 1.36 per 10,000 live births and stillbirths.[11] Its etiology is purported to be non-genetic in origin. The proposed hypothesis is (a) vascular disruption of the right lateral fold allowing the abdominal contents to eviscerate and (b) occlusion of the omphalomesenteric artery with resultant weakness of the abdominal and rupture, in utero.[12], [13], [14]
In-utero diagnosis is an important consideration in preventing morbidity and mortality.[4], [7], [13] In our series, the antenatal diagnosis was not present in any of the patients owing to (a) lack of routine antenatal ultrasound facilities, (b) lack of IEC (information education and communication), and also (c) factors related to the Covid-19 pandemic (lockdown, restrictions, fear visiting medical facilities), etc.
Gastroschisis classically presents with small, underdeveloped abdominal cavities due to the evisceration of the viscera (intestines). Also, malrotation of the intestines is its important constituent.[15] The eviscerated and uncovered bowel is exposed to amniotic fluid in utero causing it to become inflamed, edematous, thickened, and sometimes matted bowel loops with overlying peel over the serosal surface causing difficulty in its reduction into the abdominal cavity (46.55% in our series).[12], [15], [16]
The incidence of Gastroschisis was slightly higher in males in our study, which was similar to other series.[2] The percentage of premature patients (63.79%) in our series was also concurrent with other reports from the West.[2]
Associated congenital anomalies in Gastroschisis may be as high as 30%.[1] These include undescended testes, congenital cardiac anomalies, trisomy 18, Mobius syndrome, and Kallmann syndrome. Associated abnormalities were present in 24% of cases in a series from the Asian subcontinent.[6], [15] Owen A et al divided gastroschisis into simple or complex types according to the presence of additional bowel damage (perforation or atresia) to direct the operative technique.[3] These abdominal wall defects are often diagnosed antenatally and ideally, these neonates should be delivered by elective cesarean section in a center where surgery can be performed, or transferred immediately after birth. In contrast to the developed nations, these defects are encountered postnatally at most primary health care centers in India and then referred to tertiary referral institutes. We are a high-volume tertiary center with 58 cases per year, while reports from central Asia suggest approximately 3 cases/per year.[1]
The principles of management of gastroschisis are (i) reduction of the viscera safely, (ii) evaluation for viscero-abdominal disproportion, (iii) closure of the abdominal wall defect with an acceptable cosmetic appearance, (iv) nutritional support, and (v) management of associated anomalies (necrosis, atresia of bowel) or complications.[7] Surgical management of gastroschisis is variable and depends on the condition of the neonate and the eviscerated contents with an assessment of the degree of abdominal wall tension before deciding the type of repair.[12] Surgical closure of the abdominal wall is either primary or staged closure. Operative primary reduction with sutured fascial defect closure is the reference standard operative procedure.[4], [7] Operative staged reduction is performed either by skin flap closure or suturing a synthetic material (using a silo) to the enlarged defect and delayed defect closure. A staged procedure is a salvage approach when reduction is considered unsafe because of VAD which may result in abdominal compartment syndrome. Silo may be silastic or Teflon or spring-loaded silo (in NICU) where slow reduction of eviscerated content is done (1-2 times/day) followed by prosthesis removal and surgical closure of defect (at 7-10 days).[4]
Non-operative ward reduction was not performed in our series as none of the patients met the selection criteria suggested by Bianchi et al.[4], [8] Also, staged reduction and delayed defect closure using preformed silos were contemplated in 8.62% of cases in contrast to 33% of all cases in the United Kingdom.[7]
Studies from the West indicate that the presence of NEC (with or without a definitive diagnosis) was keenly observed by the treating physicians.[2] In our study, only cases with bowel perforation with or without NEC which had developed in two patients were recorded. While the survival percentage of these patients had reached as high as 91 to 97%, this was less than half (44.83%) in our study.[2] Birth weight was an important factor affecting the outcomes, none of the patients less than 1500 g survived in our series. Although statistically insignificant, birth weight was lower in the mortality group than in survivors.[2]Among the patients with associated malformations in our series, there was only one survivor with associated ileal atresia (primary skin flap closure with loop ileostomy). This was low as compared to the survival rates in a study published 3 decades ago.[2]. The outcomes were better as compared to a recent study from Iran with only 25% survival rate.[17]
In our study, the outcome of preterm patients, associated intestinal atresia, presence of edematous bowel with leathery peel, patients requiring silo due to VAD, NEC, and associated malformations was poor. This is also consistent with previously published series.[2], [4], [7], [18] In our study, the primary cause of mortality was ACS and multi-organ failure, and sepsis. In our patients, the diagnosis of ACS was made on the basis of clinical signs and symptoms e.g. oliguria, abdominal distension, paralytic ileus, acute abdomen, hemodynamic instability, respiratory insufficiency with increased ventilatory pressures, and radiological findings.
Limitations of the study were that long-term outcomes could not be evaluated due to poor follow-up and resource limitations. Also, the mode of delivery was not studied in our study.
We recommend that an early referral and prompt management should be contemplated to improve the prognosis of neonates with gastroschisis.
Conclusion
Overall survival rates in our study were 44.83% which is markedly in contrast to the series published in the recent literature. Also, the mortality rate was high (85.71%) in patients with associated malformations. The outcome of preterm (premature) patients, associated intestinal atresia, presence of edematous bowel with leathery peel, and patients requiring silo due to VAD, postoperative NEC were dismal.
Notes
n1Conflicts of interest. None.
n3Author contributions: Author(s) declared to fulfill authorship criteria as devised by ICMJE and approved the final version. Authorship declaration form, submitted by the author(s), is available with the editorial office
n4Consent to Publication: Author(s) declared taking informed written consent for the publication of clinical photographs/material (if any used), from the legal guardian of the patient with an understanding that every effort will be made to conceal the identity of the patient, however it cannot be guaranteed.
References
|
|
| 1. |
Erdogan D, Azili MN, Cavusoglu YH, Tuncer IS, Karaman I, Karaman A, et al. 11-year experience with gastroschisis: factors affecting mortality and morbidity. Iran J Pediatr. 2012; 22:339-43. |
| 2. |
Snyder CL. Outcome analysis for gastroschisis. J Pediatr Surg. 1999; 34:1253-6. Available from: https://doi.org/10.1016/s0022-3468(99)90162-8. |
| 3. |
Blakelock RT, Harding JE, Kolbe A, Pease PW. Gastroschisis: can the morbidity be avoided? Pediatr Surg Int. 1997; 12:276-82. Available from: https://doi.org/10.1007/BF01372149.
|
| 4. |
Boia ES, Iacob R, Adam O, Pavel AI, Trailescu MD, Boia M, et al. Surgical treatment of gastroschisis using silimed gastroschisis container-case report. Jurnalul Pediatrului. 2006; 19:39-45.
|
| 5. |
Blane CE, Wesley JR, DiPietro MA, White SJ, Coran AG. Gastrointestinal complications of gastroschisis. AJR Am J Roentgenol. 1985; 144:589-91. Available from: https://doi.org/10.2214/ajr.144.3.589.
|
| 6. |
Abdel–Latif ME, Bolistty S, Abeywardana S, Lui K. Mode of delivery and neonatal survival of infants with gastroschisis in Australia and New Zealand. J Pediatr Surg. 2008; 43:1685-90. Available from: https://doi.org/10.1016/j.jpedsurg.2008.03.053.
|
| 7. |
Owen A, Marven S, Johnson P, Kurinczuk J, Spark P, Draper ES, et al. Gastroschisis: a national cohort study to describe contemporary surgical strategies and outcomes. J Pediatr Surg. 2010; 45:1808-16. Available from: https://doi.org/10.1016/j.jpedsurg.2010.01.036.
|
| 8. |
Bianchi A, Dickson AP, Alizai NK. Elective delayed midgut reduction—no anesthesia for gastroschisis: selection and conversion criteria. J Pediatr Surg. 2002; 37:1334-6. Available from: https://doi.org/10.1053/jpsu.2002.35003.
|
| 9. |
Luton D, de Lagausie P, Guibourdenche J, Oury J, Sibony O, Vuillard E, et al. Effect of amnioinfusion on the outcome of prenatally diagnosed gastroschisis. Fetal Diagn Ther. 1999; 14:152-5. Available from: https://doi.org/10.1159/000020910.
|
| 10. |
Komuro H, Imaizumi S, Hirata A, Matsumoto M. Staged silo repair of gastroschisis with preservation of the umbilical cord. J Pediatr Surg. 1998; 33:485-8. Available from: https://doi.org/10.1016/s0022-3468(98)90093-8.
|
| 11. |
Lausman AY, Langer JC, Tai M, Seaward PG, Windrim RC, Kelly EN, et al. Gastroschisis: what is the average gestational age of spontaneous delivery? J Pediatr Surg. 2007; 42:1816-21. Available from: https://doi.org/10.1016/j.jpedsurg.2007.07.005.
|
| 12. |
Williams T, Butler R, Sundem T. Management of the Infant with gastroschisis: A comprehensive review of the literature. Newborn and Infant Nursing Reviews. 2003; 3:55-63.
|
| 13. |
Overton TG, Pierce MR, Gao H, Kurinczuk JJ, Spark P, Draper ES, et al. Antenatal management and outcomes of gastroschisis in the U.K. Prenat Diagn. 2012; 32:1256-62. Available from: https://doi.org/10.1002/pd.3998. |
| 14. |
Curry JI, McKinney P, Thornton JG, Stringer MD. The aetiology of gastroschisis. BJOG. 2000; 107:1339-46. Available from: https://doi.org/10.1111/j.1471-0528.2000.tb11645.x. |
| 15. |
Lawson A, de la Hunt MN. Gastroschisis and undescended testis. J Pediatr Surg. 2001; 36:366-7. Available from: https://doi.org/10.1053/jpsu.2001.20718 |
| 16. |
Langer JC. Gastroschisis and omphalocele. Semin Pediatr Surg. 1996; 5:124-8 |
| 17. |
Askarpour S, Ostadian N, Javaherizadeh H, Chabi S. Omphalocele, gastroschisis: epidemiology, survival, and mortality in Imam Khomeini hospital, Ahvaz-Iran. Pol Przegl Chir. 2012; 84:82-5. Available from: https://doi.org/10.2478/v10035-012-0013-4. |
| 18. |
Cusick E, Spicer RD, Beck JM. Small-bowel continuity: A crucial factor in determining survival in gastroschisis. Pediatr Surg Int. 1997; 12:34-7. Available from: https://doi.org/10.1007/BF01194799. |