Introduction
Congenital diaphragmatic hernia (CDH) is a defect that occurs in 1:3000 newborns. The most common location for the defect is the left posterolateral region, named Bochdalek. The right side is affected in 15% of cases and is bilateral in 1-2%. Complete diaphragmatic agenesis or eventration is very rare.[1] CDH are classified according to the size of the defect (% of hemidiaphragm present): Type A: +90%, Type B: 50-75% , Type C: 25% and Type D: -10%.[2]
In 1946, Gross performed surgery in a 1 day old for the first time.[3] The conventional surgical technique for CDH is an open repair, nonetheless minimal invasive surgery offers the possibility of a laparoscopic or thoracoscopic repair. The latter approaches were mostly used for late presentation of CDH.[4]
Even though minimal invasive surgery has improved in the last years, newborns are sensitive to hypothermia and acidosis caused by CO2 insufflation. Therefore it is important to determine if newborns benefit from thoracoscopic CDH repair without significant risks. If primary closure is possible, it is performed using non-absorbable interrupted suture tied intracorporeally; extracorporeal knots can also be useful.[4]
The goal of this study is to present information regarding CDH thoracoscopic management, in a pediatric hospital, using the Reverdin needle.
Methods
It was an observational, descriptive and retrospective study. Medical records were retrieved of CDH patients who underwent thoracoscopic surgery from January to May 2021 at Dr. Roberto Gilberto Elizalde Children's Hospital located in Guayaquil, Ecuador. Studied variables were: gestational age, gender, weight, type of defect, location of defect, associated pathologies, age at surgery, duration of procedure, length of hospital stay, complications, and outcome. Results were compiled and processed using Microsoft Excel 2016. The SPSS® software, 20.0 version was used for statistical analysis.
Even though there is no consensus on when to perform surgery, certain criteria must be met (2018 Canadian Guidelines to standardize CDH Management). The urine output should be >1 ml/kg/h; FiO2 <0.5; pre-ductal O2 Sat 85-95%, normal MAP according to age, lactate <3 mmol/l and pulmonary arterial pressures lower than systemic pressure.[5] These criteria were all met in our patients.
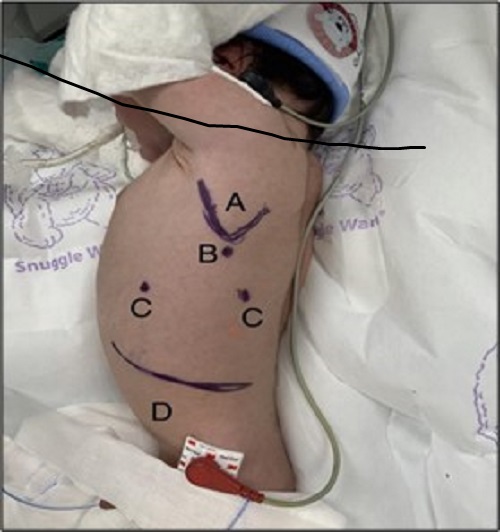
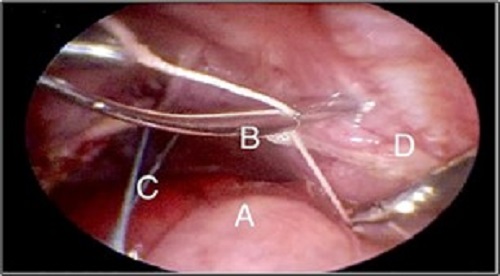
The surgical procedure begun by placing the patient on a right lateral decubitus position. (Fig. 1) An incision was made underneath the angle of the scapula, where a 3.5 mm trocar was then placed. A 3 mm camera was introduced and insufflation started (pressure between 6-8 mmHg and 1-2 l/min flow). Diagnostic thoracoscopy was performed. Two 3 mm trocars were placed in the 7th intercostal space along the anterior and posterior midaxillary lines, respectively. If hernia sac was present, it was resected with the hook electrocautery. An infra-costal incision was made, and through it a Reverdin needle was introduced, which held a 2/0 braided polyester suture. “U” stitches were made along the defect, which allowed anchoring of the posterior rim towards the abdominal wall. (Fig. 2) Traction should be applied simultaneously, to avoid tearing. Additional stitches were then placed between the first ones, ensuring adequate closure of the defect. It is important to take into account not flattening the diaphragm, to avoid the risk of recurrence. All trocars are retrieved under direct vision. As per the surgeon’s judgment, a chest tube might be placed. The postoperative cosmesis is very satisfactory. (Fig. 3)
Results
A total of 4 cases of CDH, who underwent thoracoscopic repair were included in the study, out of which 3 (75%) were male. All patients were born at term. One of them was underweight. All patients had a type B left sided CDH. The average duration of the procedure was 157.5 min. (Range: 120-240 min). Chest tube was placed in 2 patients and removed on 3rd post-op day with no complications.
One patient developed pulmonary hypertension before surgery (Surgery performed at 20th day of life), while 2 developed pulmonary hypertension in post-op period. They were all managed with nitric oxide. All the studied characteristics, intraoperative findings and outcome are summarized in Table 1
Discussion
The thoracoscopic approach for CDH repair has multiple advantages over the open technique including lower pain, rapid recovery, and lesser morbidity. Surgically it allows a wider field visualization and relatively easier herniated abdominal viscera reduction. Nonetheless, the physiologic stress caused on the newborn is something to consider, CO2 retention can cause acidosis. Other complications described are hypotension, tachycardia (especially in those with cardiac pathology), and severe pulmonary hypoplasia. These should be closely monitored as it has been proved that the severity of PH and the ability to tolerate the capnothorax will determine the success or failure of the primary thoracoscopic repair. Therefore, before surgery is performed, the neonatology and anesthesiology team should all be involved and the case should be thoroughly discussed.[6], [7]
Every neonate with CDH treated at our hospital is managed by a multidisciplinary team (neonatologist, cardiologist, anesthesiologist, and pediatric surgeon). Patients that undergo thoracoscopic repair should meet widely used criteria. FiO2 should be set lower than 50% and PH if present must not require nitric oxide at the moment of surgery.[8] Only one of our patients required NO for moderate PH, once PH was resolved surgery was performed.
Wall et al mention in their study that minimally invasive surgery can be performed safely on patients who weigh less than 3kg, despite fragility and comorbidities seen in this population. He reported an acceptable surgery conversion rate and complications were similar to the open technique.[9] The average weight of the patients who underwent thoracoscopic repair was 3075 g (range: 2400-3800g).
The survival rate is dependent on defect size. Type A: 99-100%, Type B: 96-98.8%, Type C: 91.1%, Type D: 58-80%.[7], [10], [11] Literature supports minimal invasive surgery for Types A and B.[4] In our case series all patients treated had a type B defect with a 100% survival rate. It is difficult to predict the size of the defect before surgery, but using the position of the stomach in the initial x-ray can offer some insight. An intra-abdominal stomach suggests an intact esophageal hiatus, thus a smaller defect and consequently a bigger posterior diaphragmatic rim.[7]
Shalaby et al described that the main disadvantage of conventional thoracoscopic repair is the difficulty of intracorporeal suture knot tying.[10] This difficulty is avoided with the technique described in our study. It also has the advantage of requiring a less steep learning curve. Fujishiro et al studied the duration of the procedure and found an average of 136 min (Range: 82-245).[8] Although the type of defect was not specified. In our case series the average duration was 157.5 min (Range: 120-240 min), which we believe will decrease as the surgeon's learning curve improves.
The stress caused by surgery itself is often enough to induce a pulmonary hypertension crisis.[12] This was seen in 2 (50%) of our patients and warranted NO use, with good outcome. It is important to be on the lookout for this complication, especially in the post-op setting.
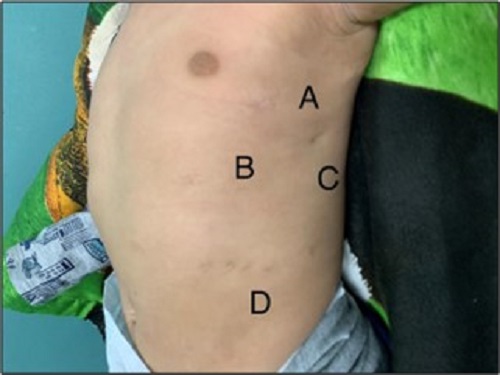
There is no consensus on whether to use a chest tube on CDH repair, some studies as Schlager et al support its use.[13] Others recommend its use but suggest its retrieval as soon as possible.[4] Chest tube was placed on 2 of our patients. Good outcomes were obtained regardless of chest tube placement. The length of hospital stay in our patients was a mean of 22.25 days (Range: 10-35 days), similar to Putnam et al.[11] One of the advantages of thoracoscopy is fewer scars. (Fig. 3)
The limitations of our study were single hospital involvement, retrospective, small patient population and short follow up period. Due to this we cannot confirm the higher risk of recurrence for CDH repaired thoracoscopically, which is described in the great majority of studies.[11], [14]Table 2 describes the comparison of our study to the literature.
We suggest further studies be performed with a larger patient population, prospective in nature, with longer follow up time and involvement of several hospitals.
Conclusion
CDH repair in newborns, performed by thoracoscopy using the Reverdin needle is a simple and reproducible technique. It avoids the difficulty that intracorporeal knots impose. Patients have to be selected according to specific criteria and there must be a multidisciplinary team approach (neonatologist, anesthesiologist, cardiologist and pediatric surgeon). We propose that the use of the Reverdin needle will increase the possibility of a successful surgery.
Notes
W: Weeks, M: Male, F: Female, D: day, PH: Pulmonary Hypertension, LPL: Left Posterolateral, - No, + Yes
n1Conflicts of interest. None.
n3Author contributions: Author(s) declared to fulfill authorship criteria as devised by ICMJE and approved the final version. Authorship declaration form, submitted by the author(s), is available with the editorial office.
n4Consent to Publication: Author(s) declared taking informed written consent for the publication of clinical photographs/material (if any used), from the legal guardian of the patient with an understanding that every effort will be made to conceal the identity of the patient, however it cannot be guaranteed.
References
|
|
| 1. |
García-Posada R, Gómez O, Martínez JM, Puerto B, Gratacós E. [Congenital diaphragmatic hernia: prognostic criteria and current status of prenatal treatment]. Guía clínica Diagnóstico Prenat. 2012; 23:126-33. Article in Spanish. |
| 2. |
Mesas Burgos C, Frenckner B, Harting MT, Lally PA, Lally KP. Congenital Diaphragmatic Hernia Study Group. Congenital diaphragmatic hernia and associated omphalocele: a study from the CDHSG registry. J Pediatr Surg. 2020; 55:2099-2104. https://doi.org/10.1016/j.jpedsurg.2019.10.056. |
| 3. |
Gross RE. Congenital hernia of the diaphragm. Am J Dis Child. 1946; 71:579-92.
|
| 4. |
Schneider A, Becmeur F. Pediatric thoracoscopic repair of congenital diaphragmatic hernias. J Vis Surg. 2018; 28:43. https://doi.org/10.21037/jovs.2018.02.03.
|
| 5. |
Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, Bailey M, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ. 2018; 190:E103-E112. https://doi.org/10.1503/cmaj.170206.
|
| 6. |
Choudhry M, Rusu S, Brooks P, Ogundipe E, Chuang SL. Thoracoscopic repair of congenital diaphragmatic hernia in preterm neonate at 1 kilogram. European J Pediatr Surg Rep. 2021; 9:e13-e16. https://doi.org/10.1055/s-0040-1721473.
|
| 7. |
Gomes Ferreira C, Kuhn P, Lacreuse I, Kasleas C, Philippe P, Podevin G, et al. Congenital diaphragmatic hernia: an evaluation of risk factors for failure of thoracoscopic primary repair in neonates. J Pediatr Surg. 2013; 48:488-95. https://doi.org/10.1016/j.jpedsurg.2012.09.060.
|
| 8. |
Fujishiro J, Ishimaru T, Sugiyama M, Arai M, Suzuki K, Kawashima H, et al. Minimally invasive surgery for diaphragmatic diseases in neonates and infants. Surg Today. 2016; 46:757-63. https://doi.org/10.1007/s00595-015-1222-3.
|
| 9. |
Wall JK, Sinclair TJ, Kethman W, Williams C, Albanese C, Sylvester KG, et al. Advanced minimal access surgery in infants weighing less than 3kg: A single center experience. J Pediatr Surg. 2018; 53:503-7. https://doi.org/10.1016/j.jpedsurg.2017.05.006.
|
| 10. |
Shalaby R, Gabr K, Al-Saied G, Ibrahem M, Shams AM, Dorgham A, et al. Thoracoscopic repair of diaphragmatic hernia in neonates and children: a new simplified technique. Pediatr Surg Int. 2008; 24:543-7. https://doi.org/10.1007/s00383-008-2128-6
|
| 11. |
Putnam LR, Tsao K, Lally KP, Blakely ML, Jancelewicz T, Lally PA, et al. Minimally Invasive vs Open Congenital Diaphragmatic Hernia Repair: Is There a Superior Approach? J Am Coll Surg. 2017; 224:416-22. https://doi.org/10.1016/j.jamcollsurg.2016.12.050
|
| 12. |
Chandrasekharan PK, Rawat M, Madappa R, Rothstein DH, Lakshminrusimha S. Congenital Diaphragmatic hernia - a review. Maternal Health Neonatol Perinatol. 2017; 11:6. https://doi.org/10.1186/s40748-017-0045-1.
|
| 13. |
Schlager A, Arps K, Siddharthan R, Clifton MS. Tube thoracostomy at the time of congenital diaphragmatic hernia repair: Reassessing the risks and benefits. J Laparoendosc Adv Surg Tech A. 2017; 27:311-7. https://doi.org/10.1089/lap.2016.0233. |
| 14. |
Liu W, Feng S, Lou Y, Wang A. Thoracoscopic versus open repair of congenital diaphragmatic hernia: A systematic review and meta-analysis. World J Surg Surgical Res. 2019; 2: 1169. |