Introduction
Congenital intrinsic duodenal obstruction (CIDO - Duodenal atresia & stenosis) is one of the common surgical congenital anomalies in newborns and infants as characterized by repeated bilious emesis.[1], [2] Over half of the patients have associated major congenital anomalies and syndromes such as Down syndrome, congenital cardiac anomalies, and gastrointestinal anomalies.[3], [4], [5], [6] It is often associated with maternal polyhydramnios, prematurity, and low birth weight. [7]
Over the years, the prognosis of neonates and infants with CIDO has improved markedly, which is attributed to several factors such as early diagnosis, improved surgical techniques, and improved perioperative management by neonatologists, including the availability of neonatal intensive care unit (NICU), total parenteral nutrition (TPN) and neonatal anesthesia in developed countries. [3] Several factors, however, still affect the overall outcome including prematurity, low birth weight, a high incidence of associated anomalies, and reoperations.[8], [9] The aims of the study were to analyze the clinical profile including demography, clinical presentation, operative findings, postoperative complications, and the predictors of outcome of congenital intrinsic duodenal obstruction.
Methods
This is a clinical observational study comprising 67 consecutive cases of intrinsic duodenal obstruction. After IRB approval, the patients for the study were selected from the pediatric surgical unit of a tertiary care center from October 2018 to June 2020.
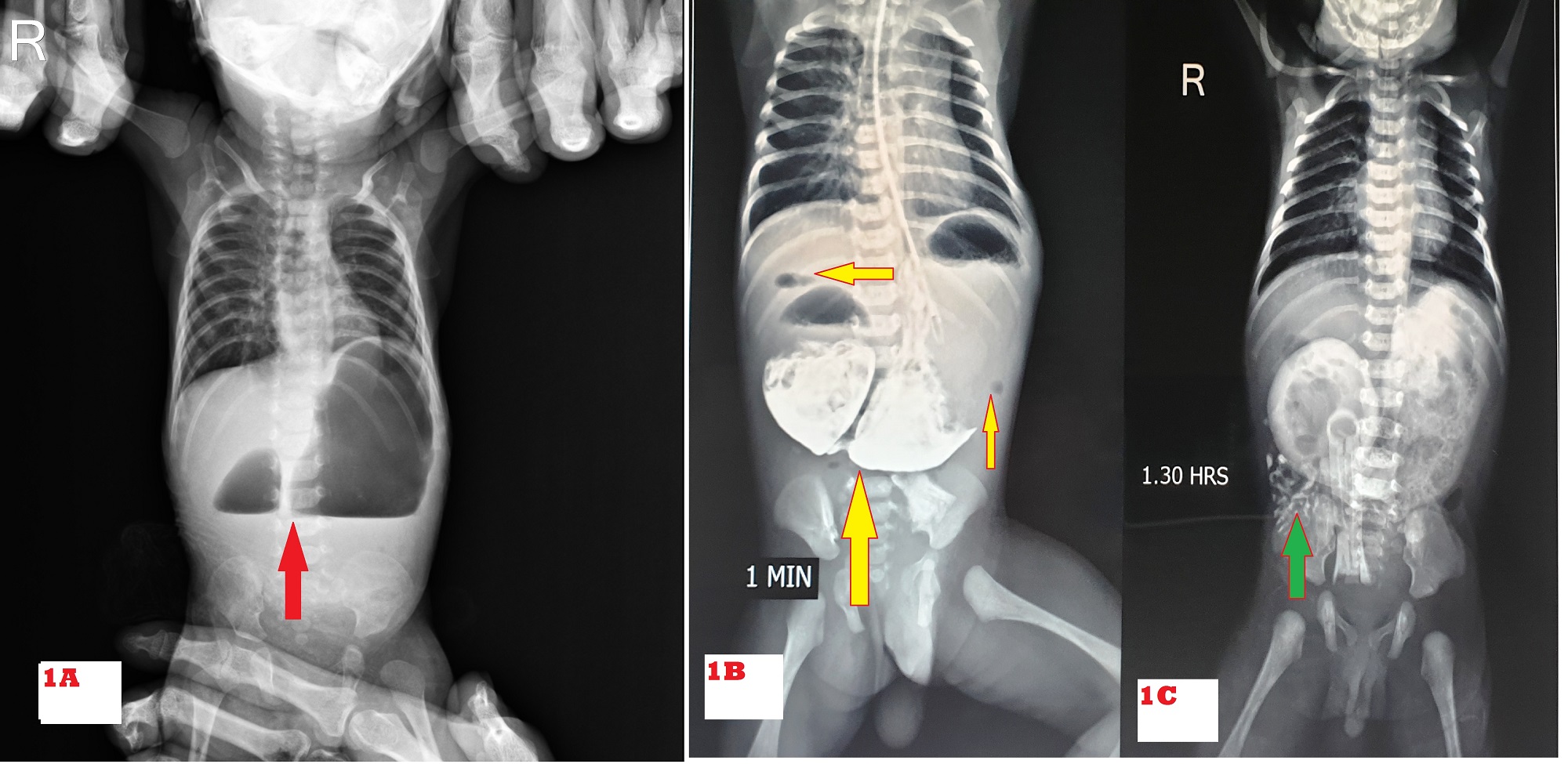
All patients (institutional & referred) with neonatal intestinal obstruction (NIO) were admitted to NICU. Patients were optimized and evaluated as per institutional protocol viz plain X-ray abdomen (typical double bubble appearance) (Fig. 1A), ultrasonography (USG) abdomen (to rule out associated malrotation, urogenital anomalies, and annular pancreas), routine blood investigations. Once the diagnosis of duodenal obstruction was made, demographic data was recorded as age at presentation, weight at presentation, birth weight, antenatal history, clinical presentation, x-ray abdomen, and baby gram findings. Bedside 2D Echo was done in all patients who had murmur on auscultation and who were hemodynamically unstable. In rest of the patients, it was done post-discharge on OPD basis. Water-soluble contrast (Gastrografin) upper gastrointestinal (UGI) study was done to confirm partial duodenal obstruction due to perforated duodenal web when in doubt. UGI endoscopy was done in one patient (presented with a history of ingestion of foreign body, the perforated duodenal web was incidental finding). Down's syndrome was suspected based on clinical features and karyotyping was done for affordable patients. Exploratory laparotomy was performed in all patients, intraoperative type of duodenal atresia and associated anomaly if the present were noted. Either kimura’s duodenoduodenostomy (KDD) or side to side duodenoduodenostomy was performed for duodenal atresia. Excision of web and Heineke-Mikulicz duodenoplasty (EW&HMD) was performed for the duodenal web. Other procedures were performed for associated congenital anomalies like isolated esophageal atresia (IEA), tracheo-esophageal fistula (TEF), anorectal malformation (ARM), malrotation, and other bowel atresia. Postoperatively, the clinical course, complications, and outcome as discharged alive or expired were noted. We analyzed the predictors of outcome variables as gestational age (term ≥37 or preterm <37weeks)[10], prenatal or postnatal diagnosis of duodenal obstruction, birth weight (normal weight ≥2500 or LBW 1500-2499 or VLBW <1500grams) [11], age on presentation, associated anomalies, duration of surgery (<90 minutes or >90 minutes), extubation after surgery (on table or <24 hours or >24 hours), post-operative sepsis and vasopressors support.
Inclusion criteria: All patients with an intraoperative finding of intrinsic duodenal obstruction were included in the study.
The statistical analysis was done using Microsoft Excel (XLSTAT). The mean, range, percentage, standard deviation, and chi-square tests were analyzed. The P-value < 0.05 was considered statistically significant.
Results
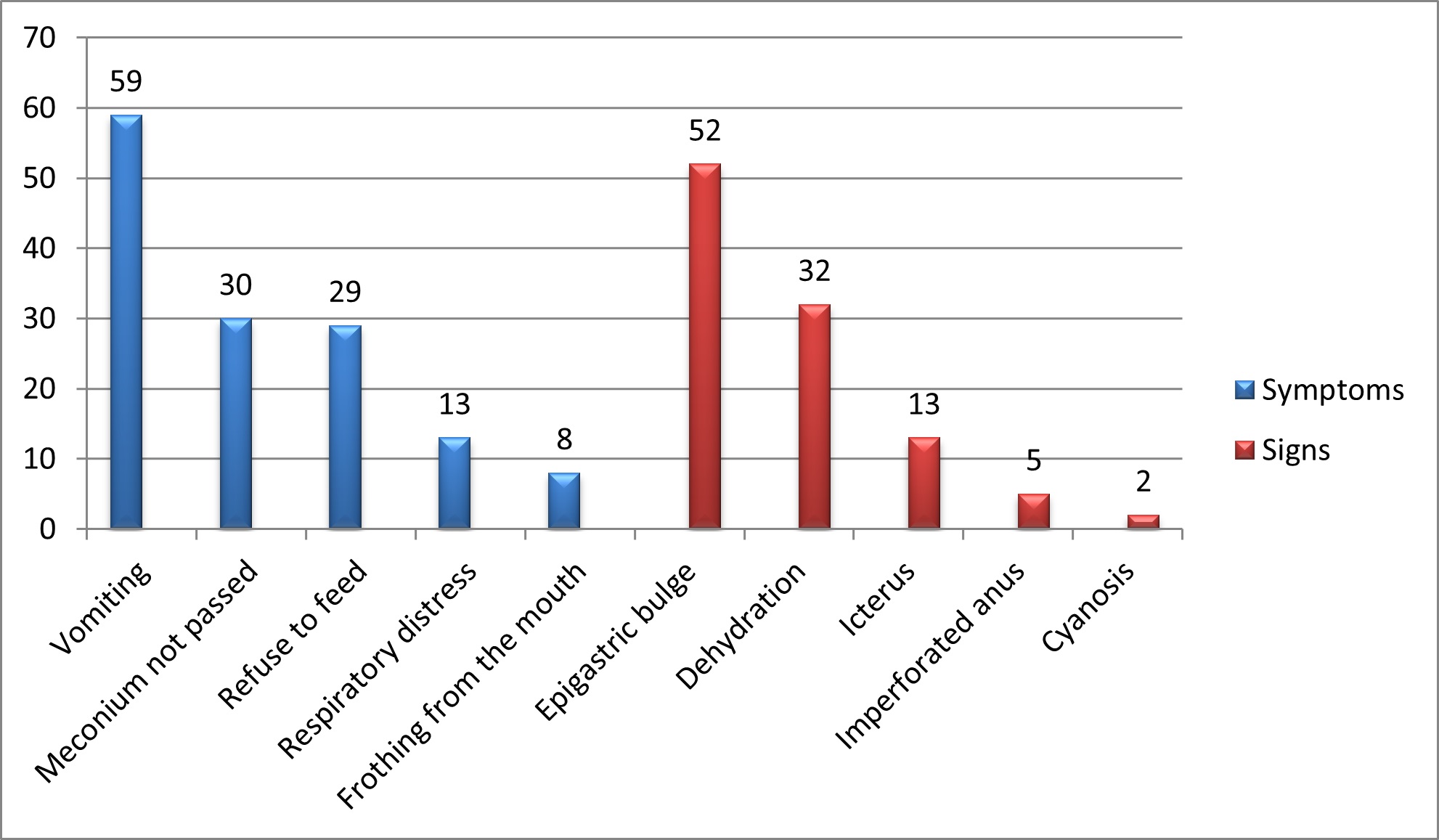
Of 67 patients, 39 were male and 28 were female with a male to female ratio of 1.4:1. The demographic characteristics of the patients are shown in Table. 1. The common presenting features on presentation were vomiting in 59 (88%) of which 44 had bilious and 15 had non-bilious vomiting, and epigastric bulge in 52 (77%) (Fig. 2). Double bubble appearance on X-ray abdomen was seen in 54 (81%) (Fig. 1A). Seven infants had distended stomach and duodenum with gas in the small bowel on abdominal x-ray. Abdominal USG color Doppler was done to rule out malrotation. Six underwent a gastrografin upper GI contrast study which gave suspicion of the perforated duodenal web (Fig. 1B, 1C). One patient underwent Upper GI endoscopy which showed windsock deformity with a small hole on the web along with a foreign body. The inability to insert stiff red rubber catheter beyond 10 cm per orally with double bubble appearance on of babygram was observed in 7 patients. A single bubble was found in 5 cases. In the patient with IEA, the diagnosis of CIDO was made during feeding gastrostomy, in which the infant feeding tube was not negotiable beyond the second part of the duodenum; moreover, the stomach and duodenum were dilated and filled with bile mixed mucus.
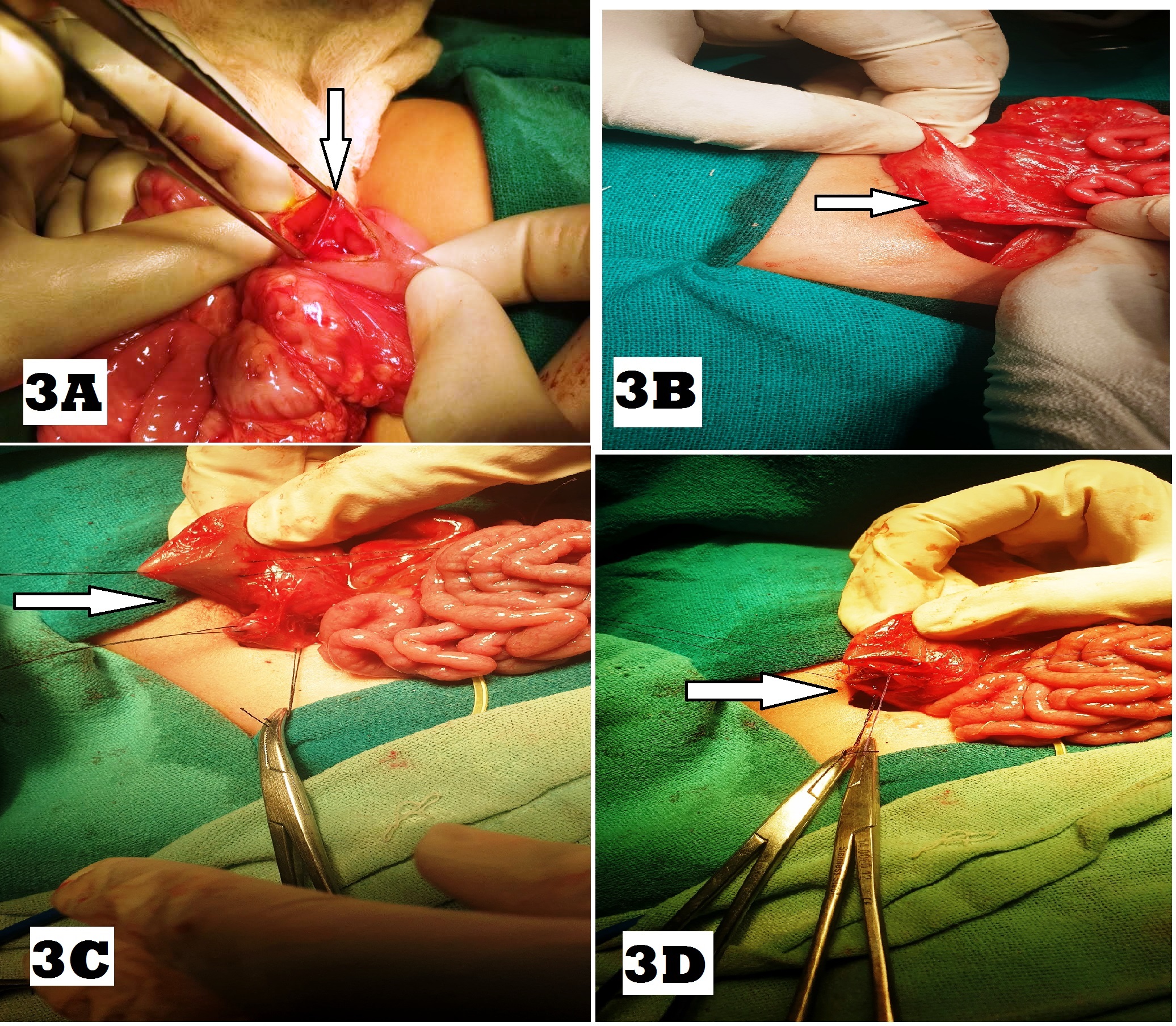
On exploratory laparotomy, type 3 duodenal atresia was found in 43(64%) followed by type 1 in 22(33%), of which 15 had web and 7 had stenosis, and type 2 which was seen in 2 (3%) patients (Fig. 3A, 3B, 3C). The location of duodenal atresia was pre-ampullary in 16 (24%) and post-ampullary in 51 (76%). The most common associated anomalies were CHD in 24 (36%) and Down’s syndrome in 19 (28%), followed by gastrointestinal in 17 (25%), urogenital anomalies in 7 (10%), and limb defects in 7 (10%), as shown in Table. 2. Three patients had triple atresia and 4 patients had VACTERL association. [12]
The operative procedures performed for duodenal atresia and associated anomalies are shown in Table. 3. The procedure performed for duodenal atresia included Kimura’s duodenoduodenostomy in 49 (73%)(Fig. 3D) followed by EW & HMD in 13 (19%) and side to side duodenoduodenostomy in 4 (6%). Duodenojejunostomy was performed in a cases as reoperation after Ladd’s procedure (1st diagnosis malrotation) on a postoperative day 8 for the missed diagnosis of the perforated duodenal web, which was suggested by upper GI study done on postoperative day 6.
Forty-two (63%) patients were discharged alive and 25 (37%) expired. Common events in the postoperative course of survivors were the start of TPN on Day 2 (Day1-Day5), NS enema on Day 3 (Day2-Day4), the passage of meconium Day 4 (Day3-Day11), NG removal on Day 6 (Day5-Day14), the start of feed on Day 7 (Day5-Day14) and discharge on Day 9 (Day7-Day16). The most common postoperative complication was sepsis in 19 (28%), followed by pneumonia in 17 (25%), functional obstruction, and wound infection in 8 (12%). None of the patients showed evidence of an anastomotic leak. The analysis of predictors of mortality is shown in Table. 4. In neonates with triple atresia and VACTERL association, the mortality was 100%.
Prenatal diagnosis: Twenty-three (34%) neonates had a suspicion of duodenal obstruction on prenatal USG, while 44 (66%) were diagnosed postnatally. The prenatal diagnosis of CIDO was not a significant predictor of mortality with a p-value >0.05.
Age at presentation: Twenty-seven (40%) presented within 24-72 hrs, 18 (27%) between 3-7days, 16 (24%) between 8- 28days and 6 (9%) presented after 28days of life. Age at presentation was not a significant predictor of mortality with a p-value >0.05.
Gestational age: Most babies were preterm 40 (60%) <37weeks while 27 (40%) were born at term. The mortality was 5 (15%) in term neonates while 21 (52%) in preterm babies. The mortality was significantly high amongst preterm neonates with a p-value of <0.05.
Birth weight: Eleven (16%) neonates had normal weight ( >2500gm), 49(73%) had LBW (1500-2499gm), and 7 (11%) had VLBW (<1500gm). The mortality was only 1(9%) for normal weight, 17 (35%) in LBW, and 7(100%) in VLBW babies. In VLBW and LBW babies mortality was high compared to normal weight with a p-value <0.05.
Associated anomalies: Amongst major associated anomalies, the mortality was 17(70%) for CHD, and 8(100%) for TEF/IEA, independently and/ or in combination with Down’s syndrome (p-value <0.05).
Duration of surgery: The patients who required a longer duration of surgery (>90 minutes) had more mortality compared to patients requiring less duration (p-value <0.05).
Extubation after surgery: Forty-one (61%) patients were extubated after completion of the procedure, 9 (14%) within 24hours, and 17(25%) after 24 hours or could not be extubated at all with respective mortality as 8(19%), 3(33%), and 13(76%). Mortality was found to be high in patients who were extubated 24 hours after surgery (or could not be extubated at all) (p-value <0.05).
Sepsis: The mortality was 16(84%) in patients who developed sepsis compared to 9(18%) who did not have sepsis. The patients with sepsis were associated with significantly higher mortality (p-value <0.0001). Infection with Gram-negative, Gram-positive, and fungal organisms was seen in 16 (84.2%), 2 (12.5%), and 1 patient, respectively.
Vasopressors support: Twenty-seven (40%) patients required higher doses of dopamine and dobutamine with/without adrenaline for hemodynamic instability, and 40 (60%) did not. The mortality was 24(88%) and 1(2.5%) in those who required vasopressors and those who did not. The patients requiring vasopressors were associated with significantly higher mortality (p-value <0.0001).
Discussion
The incidence of CIDO is 1 in 5000 to 10,000 live births with a male preponderance. [6], [13], [14] Error in the recanalization of obliterated duodenal lumen due to rapid proliferation of epithelium during the sixth week of development thought to be the primary cause. [2] Usually, the atresia, as well as the stenosis, is limited to the first and second part of the duodenum. They are relatively uncommon proximal to the ampulla of Vater, the most common site being just at the ampulla. Multiple atresias are less frequent. [6]
In our study, The CIDO was found more in males, the same trend was reported by other series.[1], [4], [5], [7] Twenty-five (37%) patients in our study, had a history of maternal polyhydramnios, the same was observed by other authors. [3], [7] The age of presentation varies depending upon whether the obstruction is partial or complete. Duodenal atresia presents earlier as compared to duodenal stenosis or perforated duodenal web/diaphragm [15], the same was observed in this series. Girvan and Stephens documented LBW in more than fifty percent of neonates with duodenal obstruction [16], the same was found in our study. Our series had prematurity in 40(60%) babies in accordance with study by Al-Salem et al. [17]
Preoperative X-ray abdomen showing a double bubble sign is diagnostic of duodenal obstruction. It was sufficient to diagnose 54 (81%) patients in this study. Duodenal web with hole (centric or eccentric) was diagnosed by gastrografin meal follow-through in 6 patients and in one by UGI endoscopy.
In our study, type 3 duodenal atresia was the commonest type (64%) followed by type 1 (33%) and type 2 (3%); similar was observed by Gupta et al. [19] and Garg et al. [20] But in other series, type 1 duodenal atresia was the frequent type of duodenal atresia. [1], [3], [4], [5], [7], [8], [9], [16], [17]
Although survival of duodenal atresia corrected surgically has been more than 95% according to western data published recently by Bethel et al. [20], in this study it was 63%. Survival of patients in Gupta et al. was 61%, which is comparable to our study. [18] In most of the developed countries, LBW, VLBW, preterm, gestational age in surgical newborns has become an obsolete parameter in the prognostic risk categorization. However, in developing countries LBW and prematurity still remain important determinants for neonatal surgical mortality (NSM).
Most of the studies failed to show a causal relationship between age at presentation, delay in seeking treatment, and NSM as was also the case in this study (P > 0.05). [22], [23], [24] Puri et al observed that LBW and premature babies were associated with a higher probability of NSM. [21] The same was observed in this study.
The presence of associated life-threatening anomalies is a significant predictor as these anomalies (mainly cardiac anomalies) can cause death unrelated to the anomaly. Such decompensation is precipitated by anesthesia and surgical stress. In this study, babies who had major associated congenital anomalies had significantly higher NSM independently or in combination with Down’s syndrome (p-value < 0.05). Similar was observed in other studies.[9], [21]
Neonatal stress response to surgery is the most important determinant of hormonal and metabolic changes following surgery which have a bearing on mortality and morbidity [25]. The longer duration of surgery (>90minutes) and multiple surgeries for associated surgical emergencies were significant predictors of NSM (p-value <0.05). Impaired neonatal respiratory physiology is an important risk factor for neonatal mortality. About 39% of neonates required postoperative mechanical ventilation and 25% required it for >24 h. Postoperative mechanical ventilation >24 hours had high NSM compared to infants who did not require or require <24hours support. Similar observations were observed by other studies. [21], [26] In the present study, infants with sepsis and who require vasopressors support were 28% and 40%, respectively. Of these infants, 84% and 88% respectively had NSM (p-value <0.0001). The Same was documented by other studies. [21], [22]
The limitation of this study was that karyotyping was not done in many patients due to affordability issues. Moreover, these neonates were cared by pediatric surgeons instead of neonatologists. The follow-up data collection after discharge from the hospital was not possible as most parents were of poor socioeconomic class and did not turn up for follow-up.
Conclusion
Congenital intrinsic duodenal obstruction commonly presents with bilious vomiting, epigastric fullness, and double bubble sign on plain x-ray abdomen. Type 3 duodenal atresia is the most common variety encountered. Prematurity, LBW, associated major congenital anomalies, duration of surgery >90 minutes, delayed extubation after surgery, sepsis, and vasopressors support are significant predictors of NSM in our setting.